| [New!] Resources |
| Home |
| Principal investigator |
| Group members |
| Research |
| Selected publications |
| Positions available |
| Activities |
| Directions to the lab |
| FAQ of GRAB sensors |
Selected publications
[ Featured Lab Articles | Featured Collaborative Articles | Featured Reviews or Previews | Main Research Articles | Reviews, Book Chapters and Highlights | Pre-prints | Collaborative Publication]
Featured Lab Articles
See more details

|

|

|

|

|

|

|
| Itch mechanism and rational design for treatment (2024) | OA roles in aversive learning (2024) | UDP sensors and next generation NE sensors (2024) | New generation 5-HT and DA sensors (2024) | Toolkits for neuropeptide sensors (2023) | Ado release mechanisms (2023) | First generation HA sensors (2023) |

|

|

|

|

|

|

|
| 5-HT roles in associative learning (2023) | First generation OT sensors (2023) | First generation ATP sensors (2021) | First generation eCB sensors (2021) | Vesicular transporter for UDP-glucose (2021) | First generation 5-HT sensors (2021) | Next generation DA and ACh sensors (2020) |

|

|

|

|
|

|
|
| Cholestatic itch mechanism (2019) | PARIS, a method for mapping gap junctions (2019) | First generation NE sensors (2019) | GRAB sensors development strategy (2019) | First generation DA sensors (2018) | First generation ACh sensor (2018) |
Featured Collaborative Articles
Featured Reviews or Previews

|

|

|

|

|

|

|
| Mesoscopic imaging (2024) | Photocaged derivative of saxitoxin (STX-bpc) (2024) | Sensors for in vivo detection (2024) | Sensors applications in depression (2024) | Tools for dopamine detection (2024) | Methods for probing neuropeptide transmission (2024) |
GRAB sensors review (2022) |
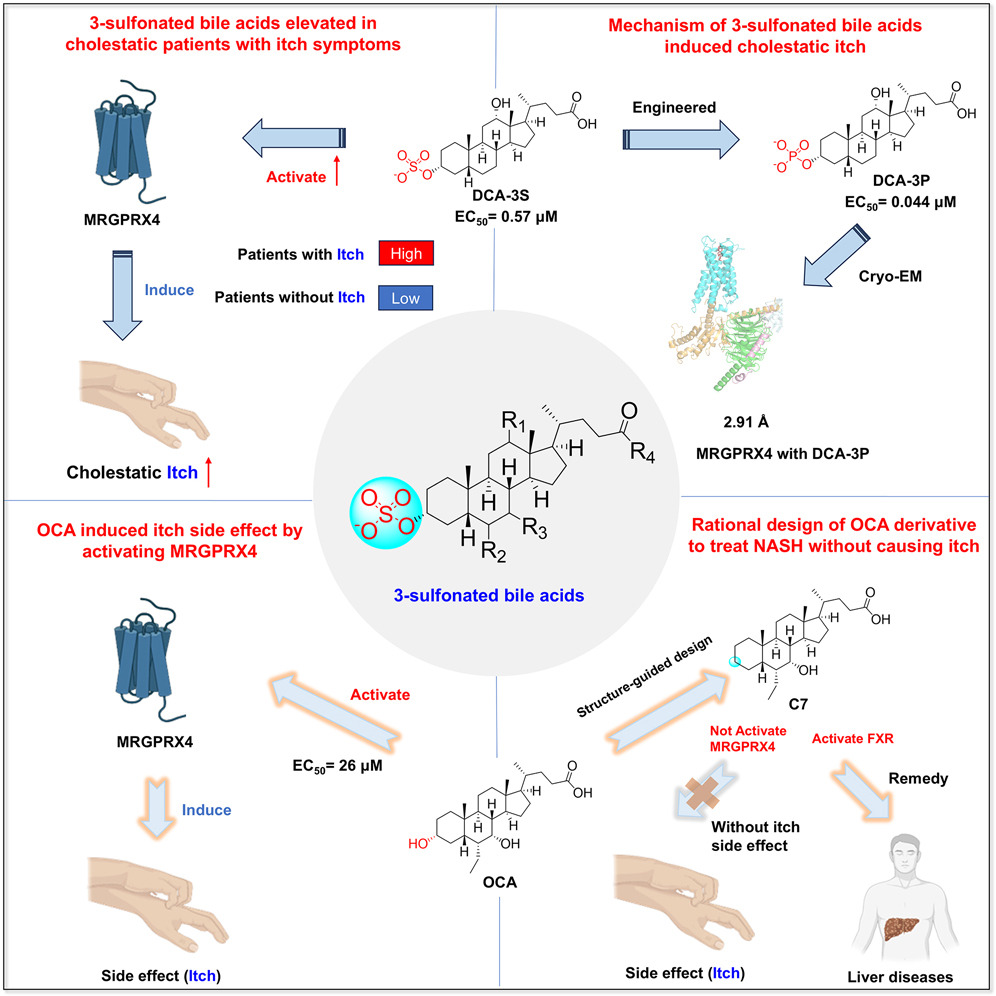 |
· Yang J., Zhao, T., Fan, J., Zou, H., Lan, G., Guo, F., Shi, Y., Ke, H., Yu, H., Yue, Z., Wang, X., Bai Y., Li, S., Liu, Y., Wang, X., Chen, Y., Li, Y.*, & Lei, X.* (2024) Structure-guided discovery of bile acid derivatives for treating liver diseases without causing itch.
Cell. DOI:10.1016/j.cell.2024.10.001.
[Full Text]
[PDF] |
|
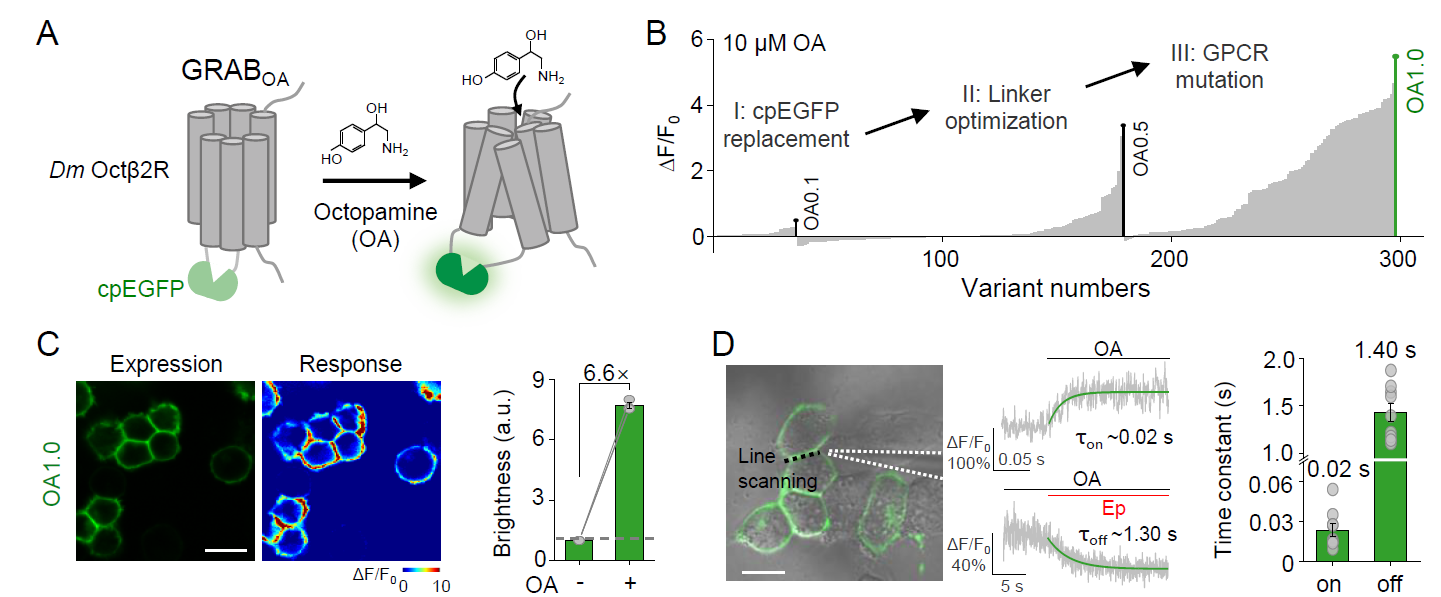 |
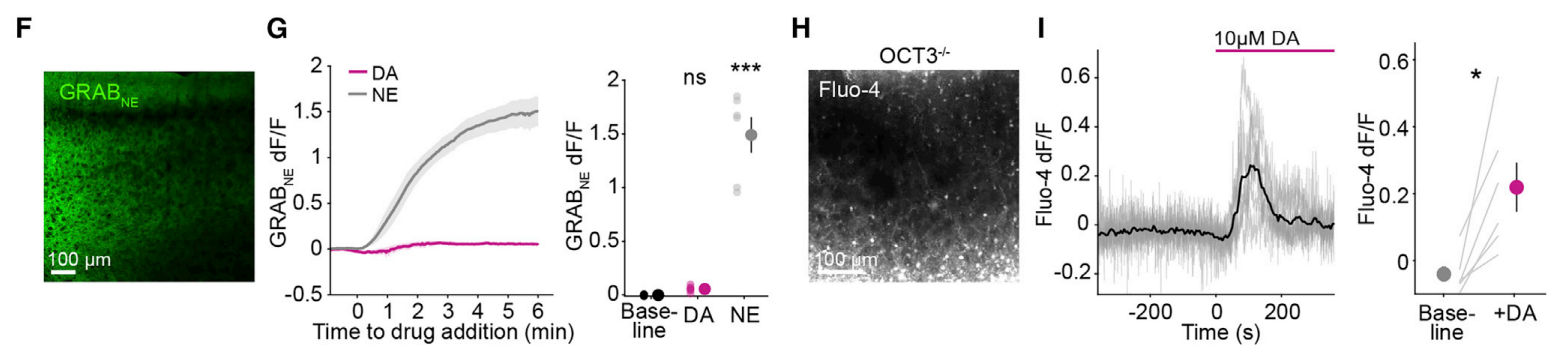
· Lv, M., Cai, R., Zhang, R., Xia, X., Li, X., Wang, Y., Wang, H., Zeng, J., Xue, Y., Mao, L., & Li, Y.* (2024). An octopamine-specific GRAB sensor reveals a monoamine relay circuitry that boosts aversive learning. National Science Review. 11(5): nwae112. [Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2024.03.09.584200 |
|
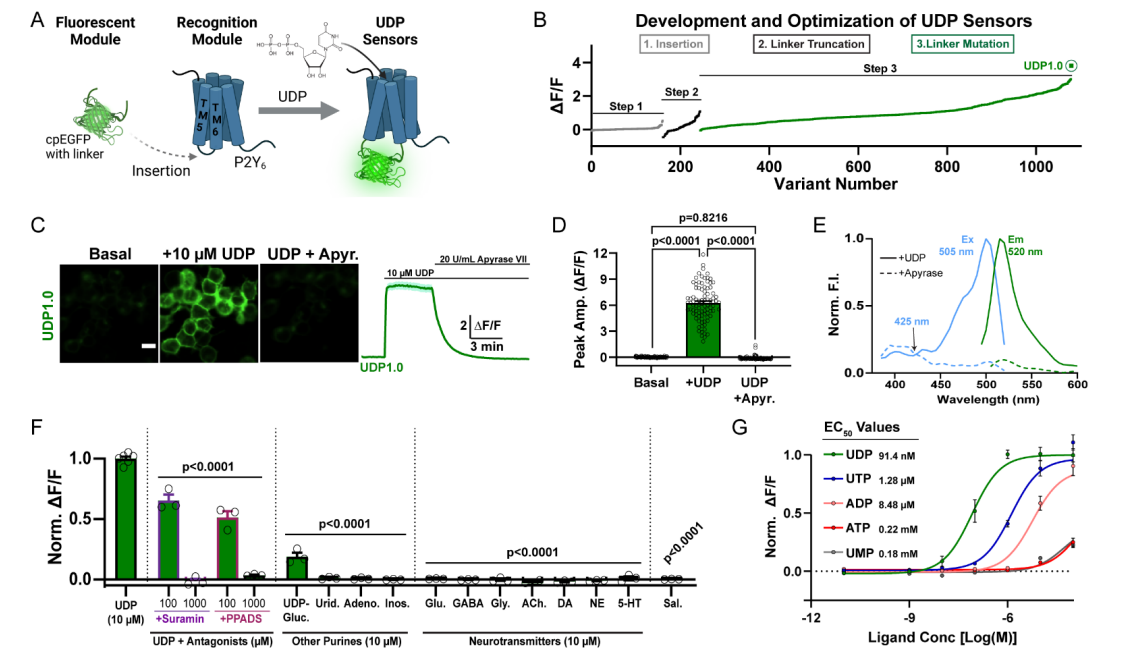 |
· Umpierre, A. D.#*, Li, B.#, Ayasoufi, K., Simon, W. L.,
Zhao, S., Xie, M., Thyen, G., Hur, B., Zheng, J., Liang, Y., Bosco, D. B., Maynes, M. A., Wu, Z.,
Yu, X., Sung, J., Johnson, A. J., Li, Y.*, & Wu, L.-J.* (2024)
Microglial P2Y6 calcium signaling promotes phagocytosis and shapes neuroimmune
responses in epileptogenesis.
Neuron.. 112(12): 1959-1977. e10.
[Full Text]
[PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2023.06.12.544691v1 |
|
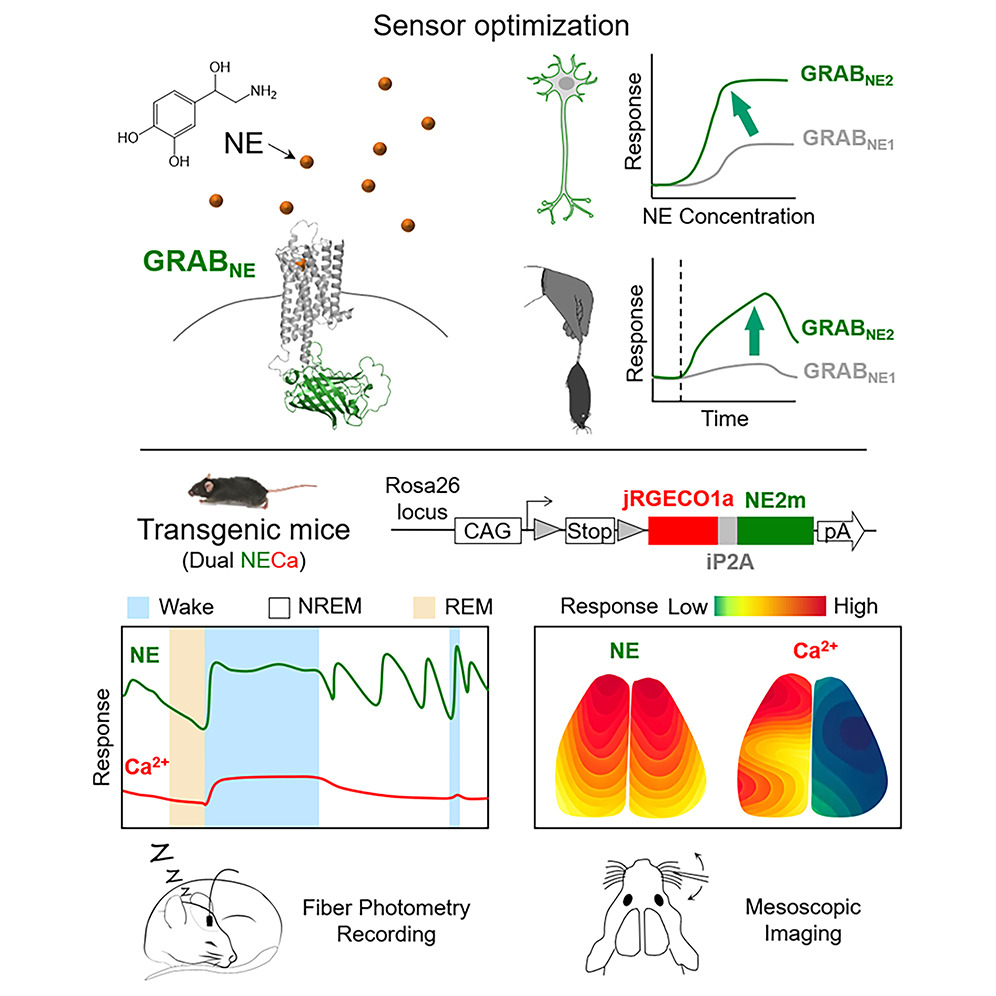 |
· Feng, J.*, Dong, H., Lischinsky, J. E., Zhou, J., Deng, F.,
Zhuang, C., Miao, X., Wang, H., Li, G., Cai, R., Xie, H., Cui, G., Lin, D.,
& Li, Y.* (2024).
Monitoring norepinephrine release in vivo using next-generation GRABNE sensors.
Neuron.. 112(12): 1930-1942. e6.
[Full Text]
[PDF] See also BioRxiv https://doi.org/10.1101/2023.06.22.546075 |
|
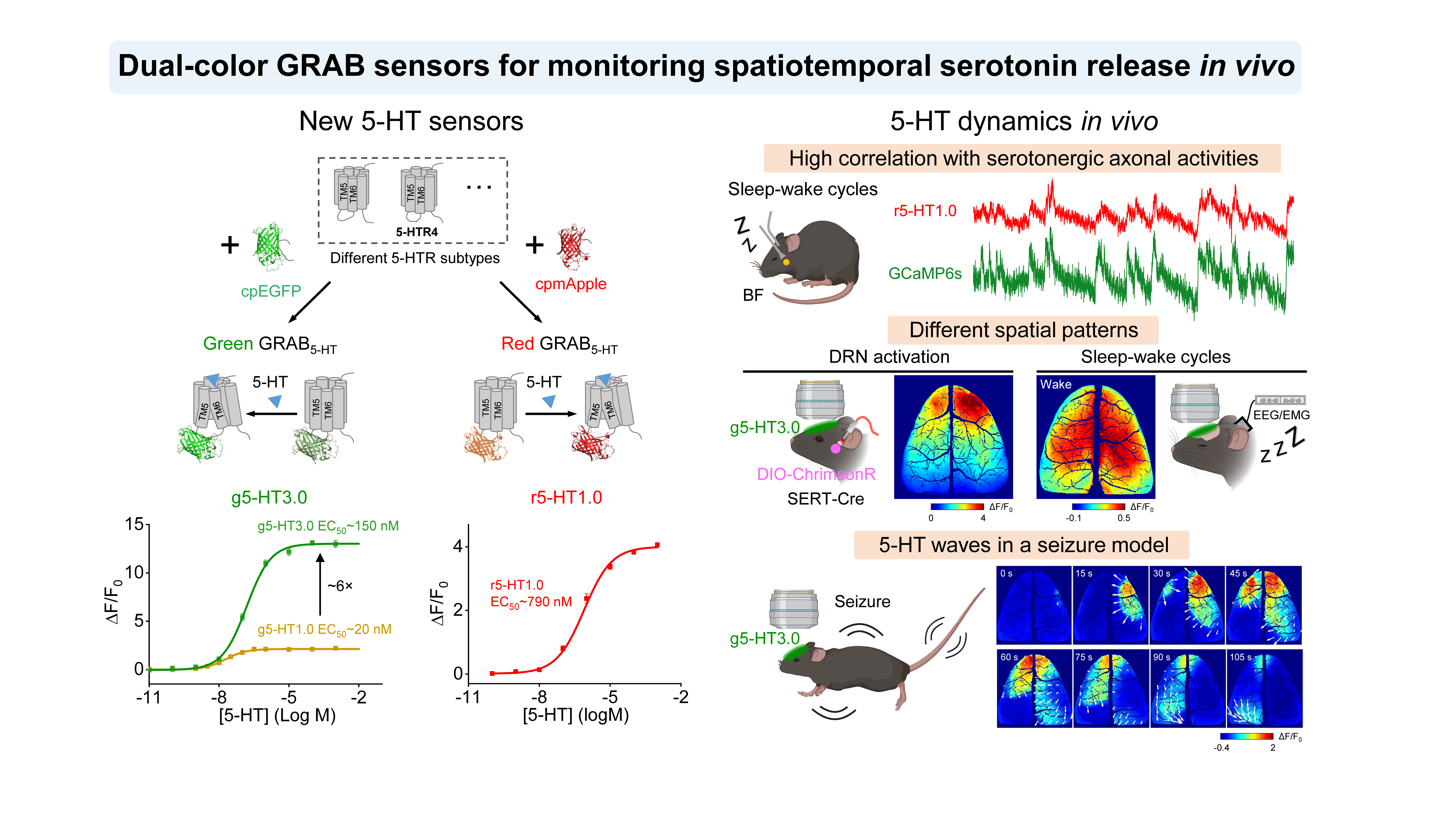 |
· Deng, F.#, Wan, J.#, Li, G., Dong, H., Xia, X., Wang, Y., Li, X., Zhuang, C., Zheng, Y., Liu, L., Yan, Y., Feng, J., Zhao, Y., Xie, H., & Li, Y.*(2024).
Improved green and red GRAB sensors for monitoring spatiotemporal serotonin release in vivo.
Nature Methods. 21(4): 692-702.
[Full Text]
[PDF] See also BioRxiv https://doi.org/10.1101/2023.05.27.542566 |
|
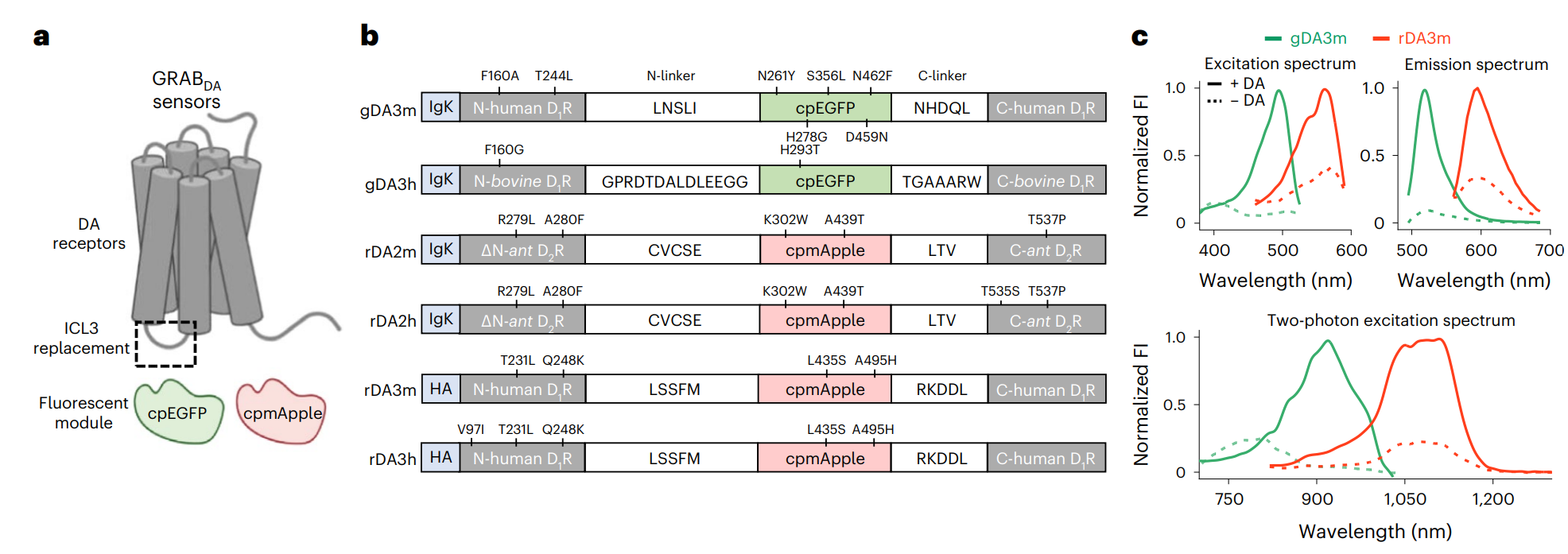 |
· Zhuo, Y.#, Luo, B.#, Yi, X., Dong, H.,
Miao, X., Wan, J., Williams, J. T., Campbell, M. G., Cai, R., Qian, T.,
Li, F., Weber, S. J., Wang, L., Li, B., Wei, Y., Li, G., Wang, H.,
Zheng, Y., Zhao, Y., Wolf, M. E., Zhu, Y., Watabe-Uchida, M., &
Li, Y.* (2024). 21(4): 680-691.
Improved green and red GRAB sensors for monitoring dopaminergic activity in vivo.
Nature Methods.
[Full Text]
[PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2023.08.24.554559v1 |
|
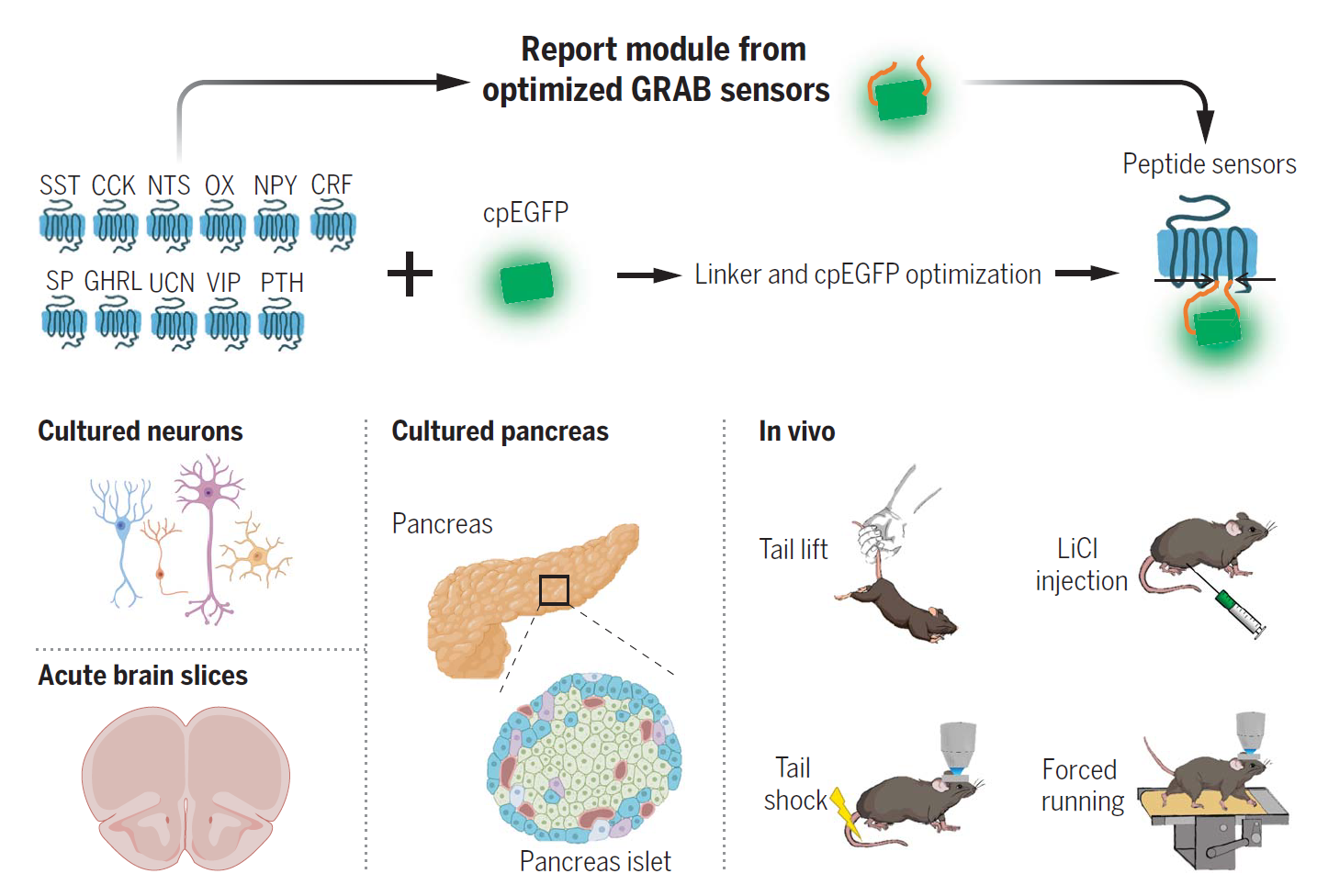 |
·
Wang, H.#, Qian, T.#, Zhao, Y., Zhuo, Y., Wu, C., Osakada, T.,
Chen, P., Chen, Z., Ren, H., Yan, Y., Geng, L., Fu, S.,
Mei, L., Li, G., Wu, L., Jiang, Y., Qian, W., Zhang, L., Peng, W., Xu, M., Hu, J.,
Jiang, M., Chen, L., Tang, C., Zhu, Y., Lin, D., Zhou, J.-N., & Li, Y.* (2023).
A tool kit of highly selective and sensitive genetically encoded neuropeptide sensors.
Science , 382(6672), eabq8173.
[Full Text]
[PDF] See also BioRxiv https://doi.org/10.1101/2022.03.26.485911 |
|
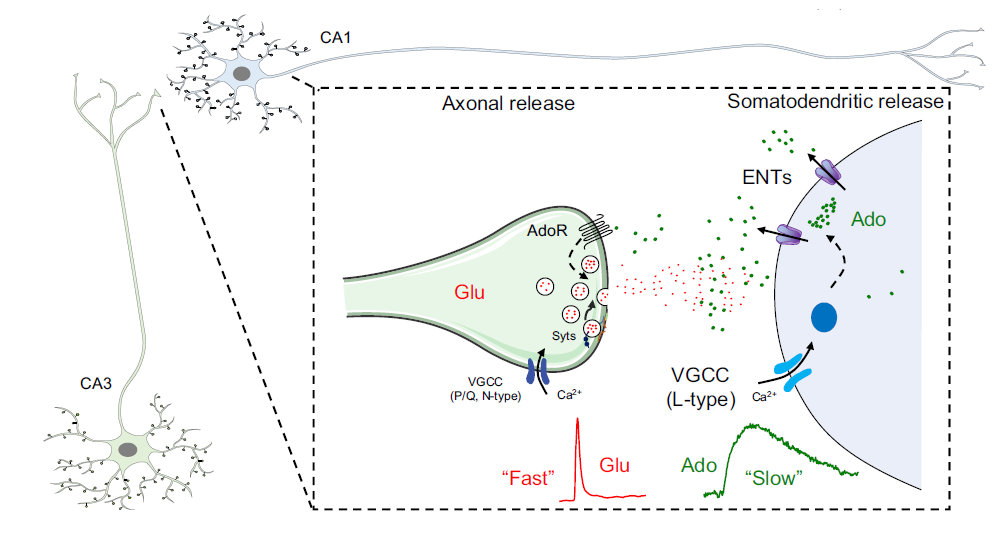 |
· Wu, Z.#, Cui, Y.#, Wang, H.#, Wu, H., Wan, Y.,
Li, B., Wang, L., Pan, S., Peng, W., Dong, A., Yuan, Z., Jing, M.,
Xu, M., Luo, M.*, & Li, Y.* (2023).
Neuronal activity-induced, equilibrative nucleoside transporter-dependent,
somatodendritic adenosine release revealed by a GRAB sensor.
Proceedings of the National Academy of Sciences, 120(14), e2212387120.
[Full Text]
[PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2020.05.04.075564 |
|
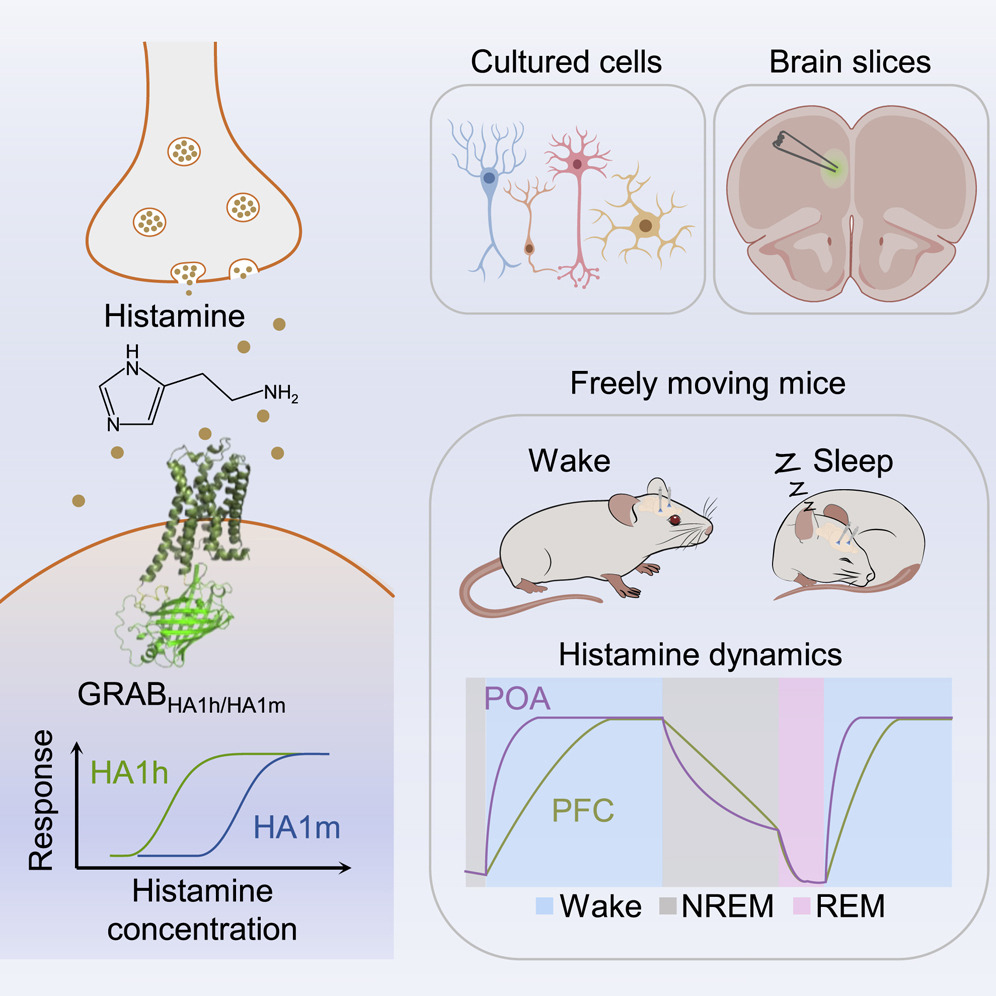 |
· Dong, H.#, Li, M.#, Yan, Y., Qian, T., Lin, Y., Ma, X., Vischer, H. F., Liu, C., Li, G., Wang, H., Leurs, R., & Li, Y.* (2023).
Genetically encoded sensors for measuring histamine release both in vitro and in vivo.
Neuron. 111(10): 1564-1576. e6.
[Full Text]
[PDF] See also BioRxiv https://doi.org/10.1101/2022.08.19.504485 |
|
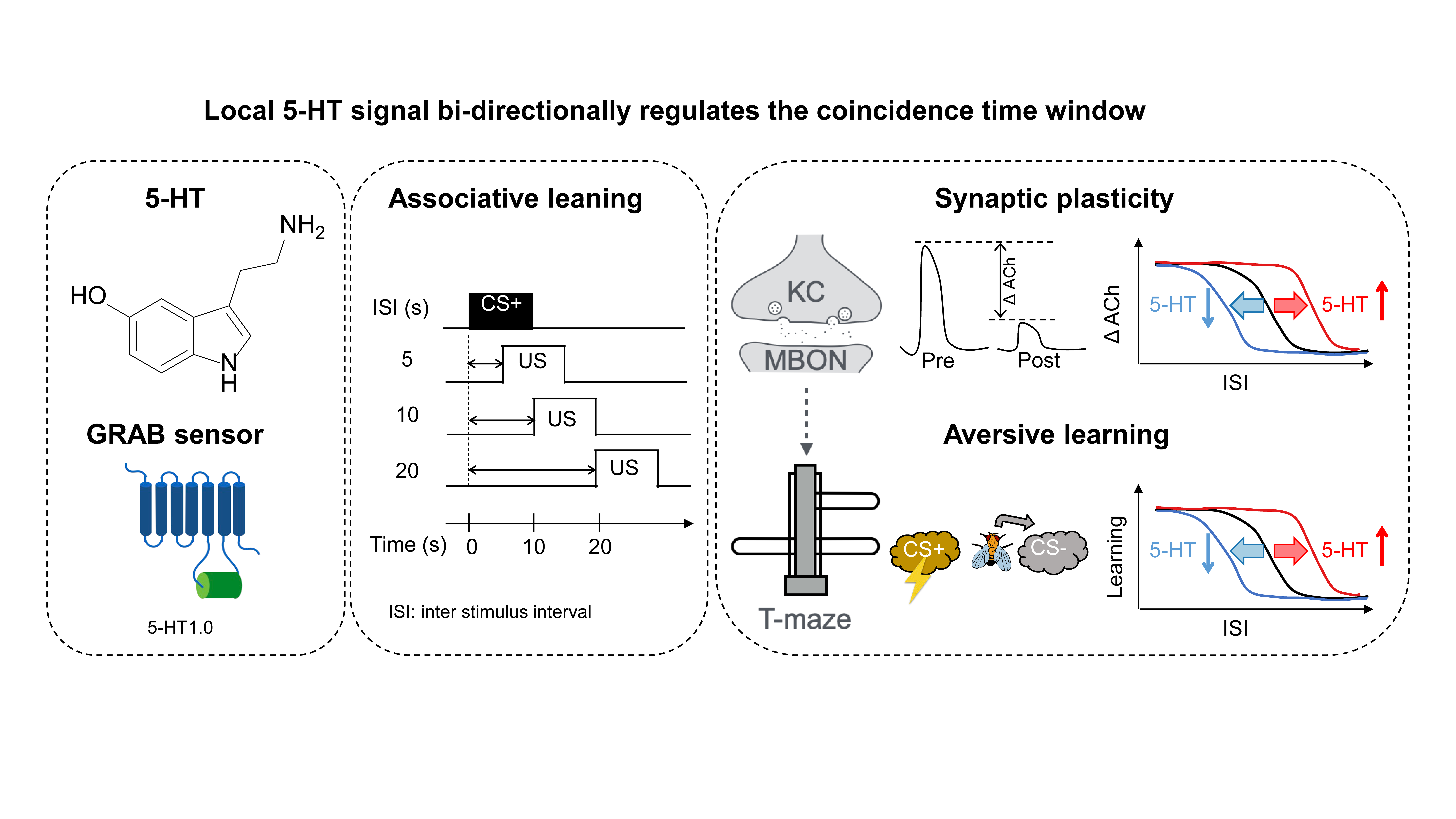 |
· Zeng, J.#*, Li, X.#, Zhang, R., Lv, M., Wang, Y., Tan, K., Xia, X., Wan, J., Jing, M., Zhang, X., Li, Y., Yang, Y., Wang, L., Chu, J., Li, Y., & Li, Y.*. (2023).
Local 5-HT signaling bi-directionally regulates the coincidence time window for associative learning.
Neuron. 111(7): 1118-1135. e5.
[Full Text]
[PDF] See also BioRxiv https://doi.org/10.1101/2022.03.27.485970 |
|
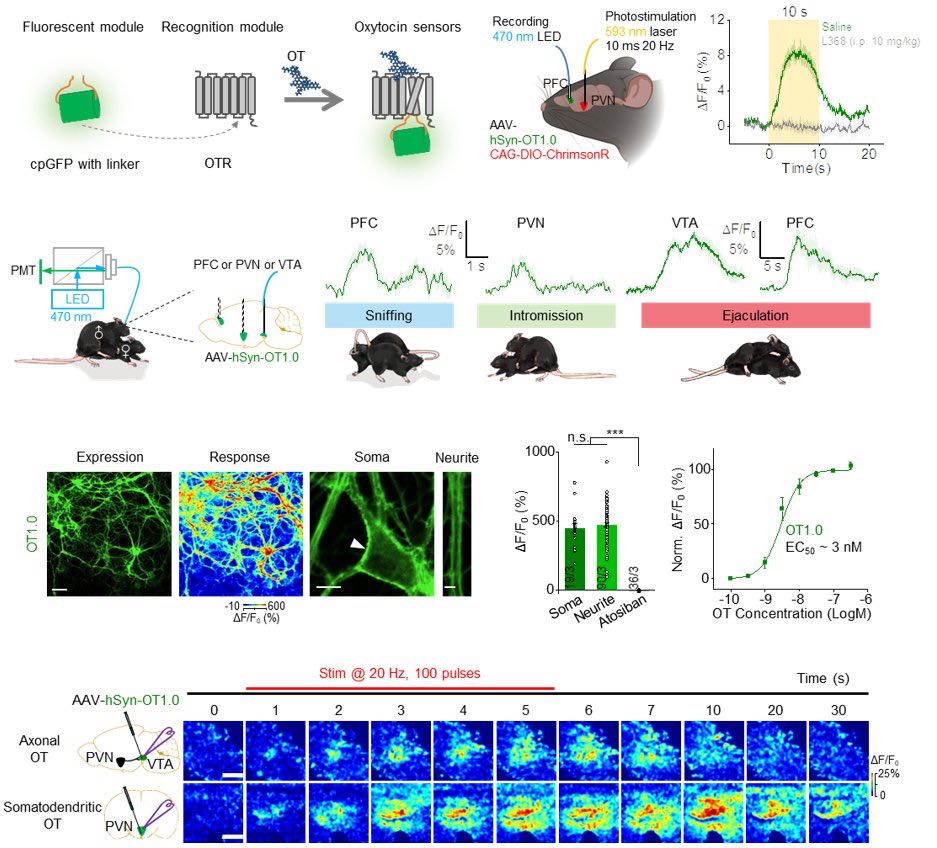 |
· Qian, T.#, Wang, H.#, Wang, P.#, Geng, L., Mei, L., Osakada, T., Wang, L., Tang, Y., Kania, A., Grinevich, V., Stoop, R., Lin, D., Luo, M., & Li, Y.* (2023).
A genetically encoded sensor measures temporal oxytocin release from different neuronal compartments.
Nature Biotechnology. 41(7): 944-957.
[Full Text]
[PDF] See also BioRxiv https://doi.org/10.1101/2022.02.10.480016 |
|
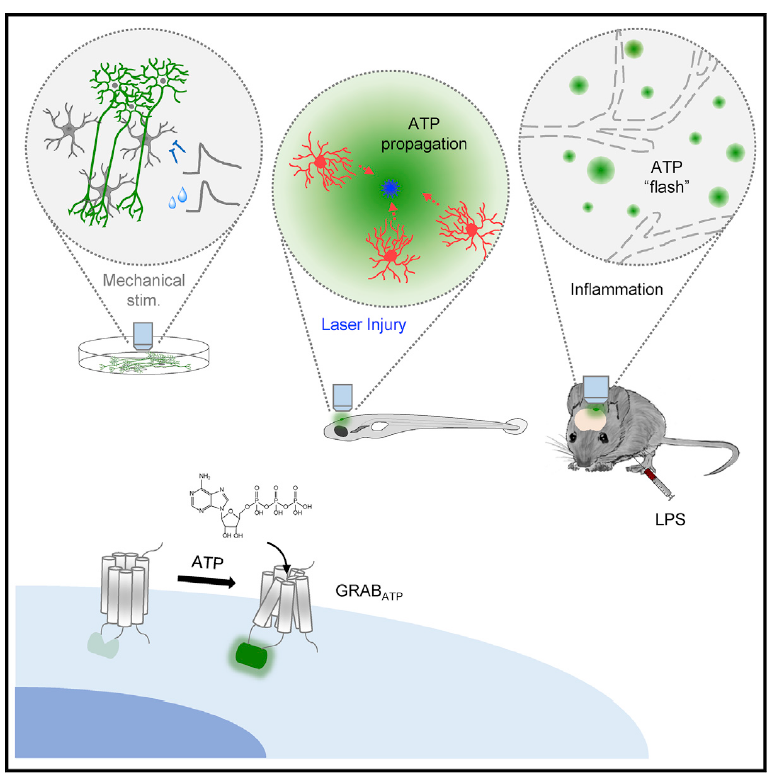 |
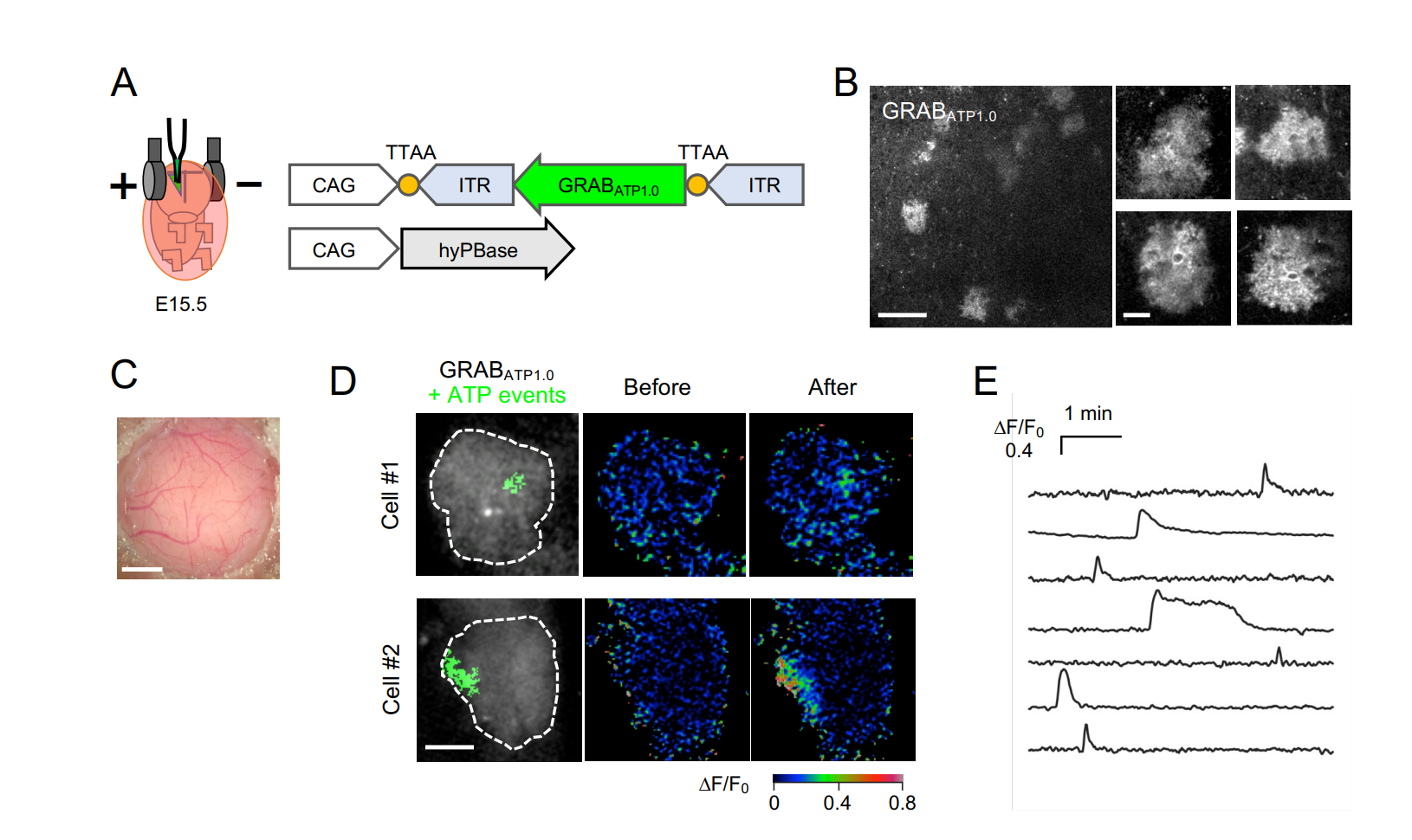
· Wu, Z.*, He, K., Chen, Y., Li, H., Pan, S., Li, B., Liu, T.,
Wang, H., Du, J., Jing, M., & Li, Y.* (2021). A sensitive GRAB sensor for detecting extracellular
ATP in vitro and in vivo Neuron, 110(5), 770-782.e775.
[Full Text]
[PDF] * See Comments Highlight by: Umpierre, A. D., Haruwaka, K., & Wu, L.-J.* (2022).
Getting a sense of ATP in real time.
Neuroscience Bulletin.
[Full Text]
[PDF] See also BioRxiv https://doi.org/10.1101/2021.02.24.432680 |
|
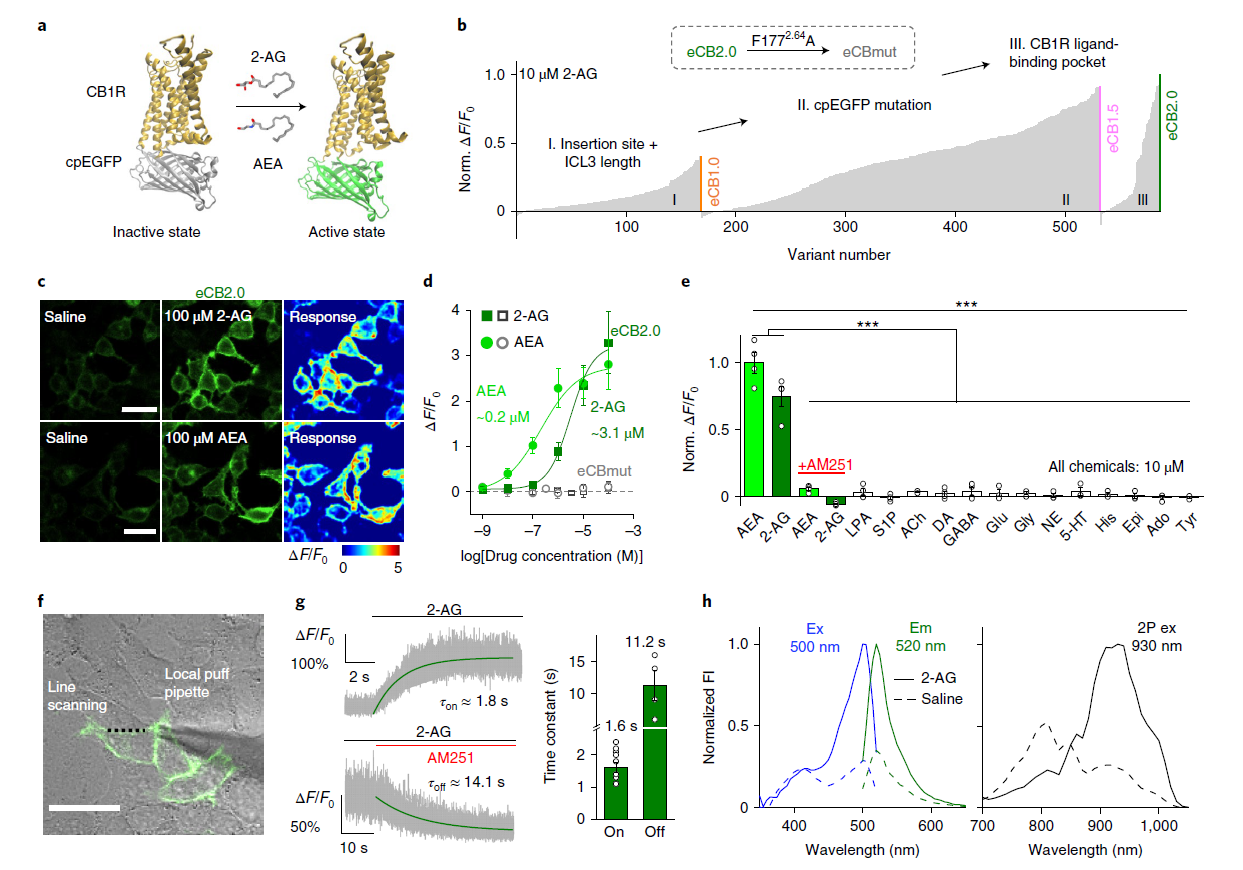 |
· Dong, A., He, K., Dudok, B., Farrell, J. S.,
Guan, W., Liput, D. J., Puhl, H. L., Cai, R., Wang, H., Duan, J., Albarran, E.,
Ding, J., Lovinger, D. M., Li, B., Soltesz, I., & Li, Y.*. (2021).
A fluorescent sensor for spatiotemporally resolved imaging of endocannabinoid dynamics in vivo.
Nature Biotechnology. 40(5): 787-798.
[Full Text]
[PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2020.10.08.329169 |
|
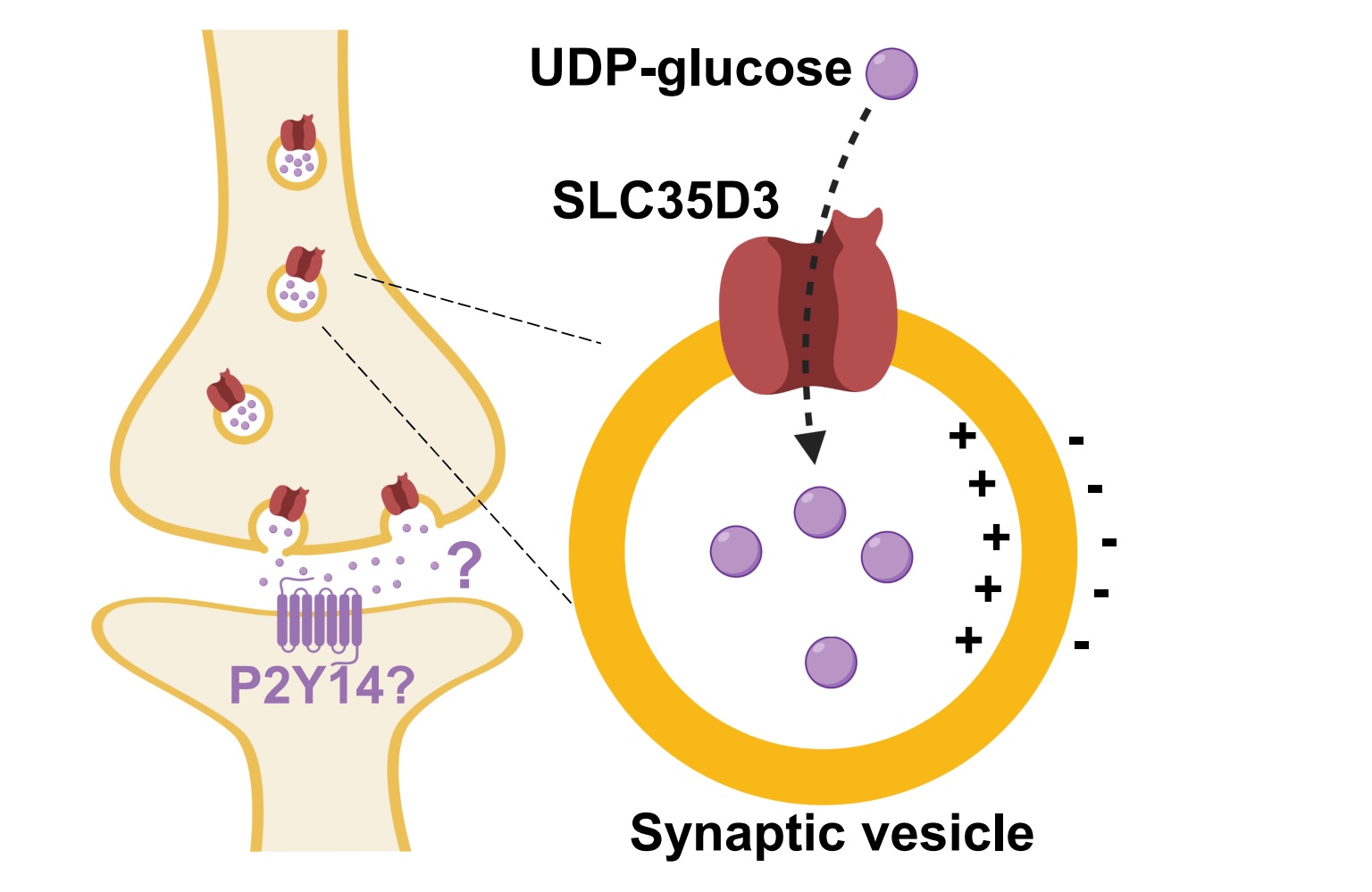 |
· Qian, C., Wu, Z., Sun, R., Yu, H., Zeng, J., Rao, Y., & Li, Y. *.
(2021). Localization, proteomics, and metabolite profiling reveal a putative vesicular transporter for UDP-glucose. eLife. 10: e65417.
https://doi.org/10.7554/eLife.65417.
[Full Text]
[PDF] See also BioRxiv https://doi.org/10.1101/2020.12.01.405605 |
|
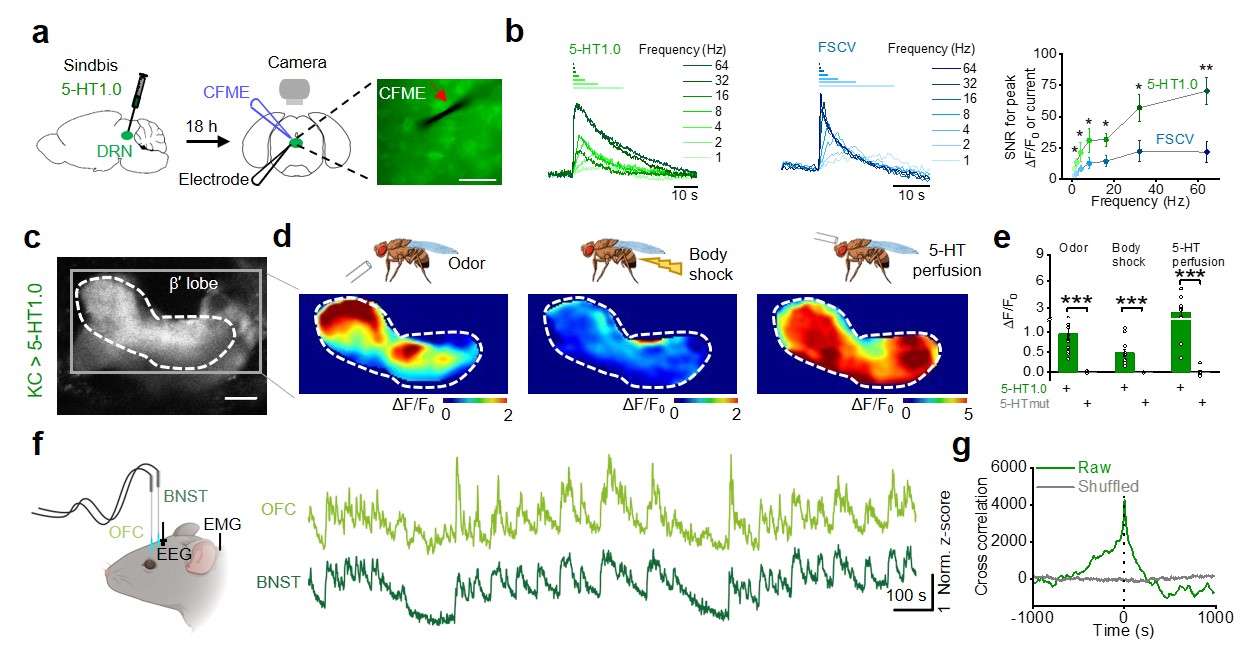 |
· Wan, J., Peng, W., Li, X., Qian, T.,
Song, K., Zeng, J., Deng, F., Hao, S., Feng,J., Zhang, P., Zhang, Y., Zou, J.,
Pan, S., Shin, M., Venton, B. J., Zhu, J. J., Jing, M., Xu, M., Li, Y.*.
(2021). A genetically encoded sensor for measuring serotonin dynamics.
Nature Neuroscience. 24(5): 746-752. https://doi.org/10.1038/s41593-021-00823-7.
[Full Text]
[PDF] See also BioRxiv https://doi.org/10.1101/2020.02.24.962282 |
|
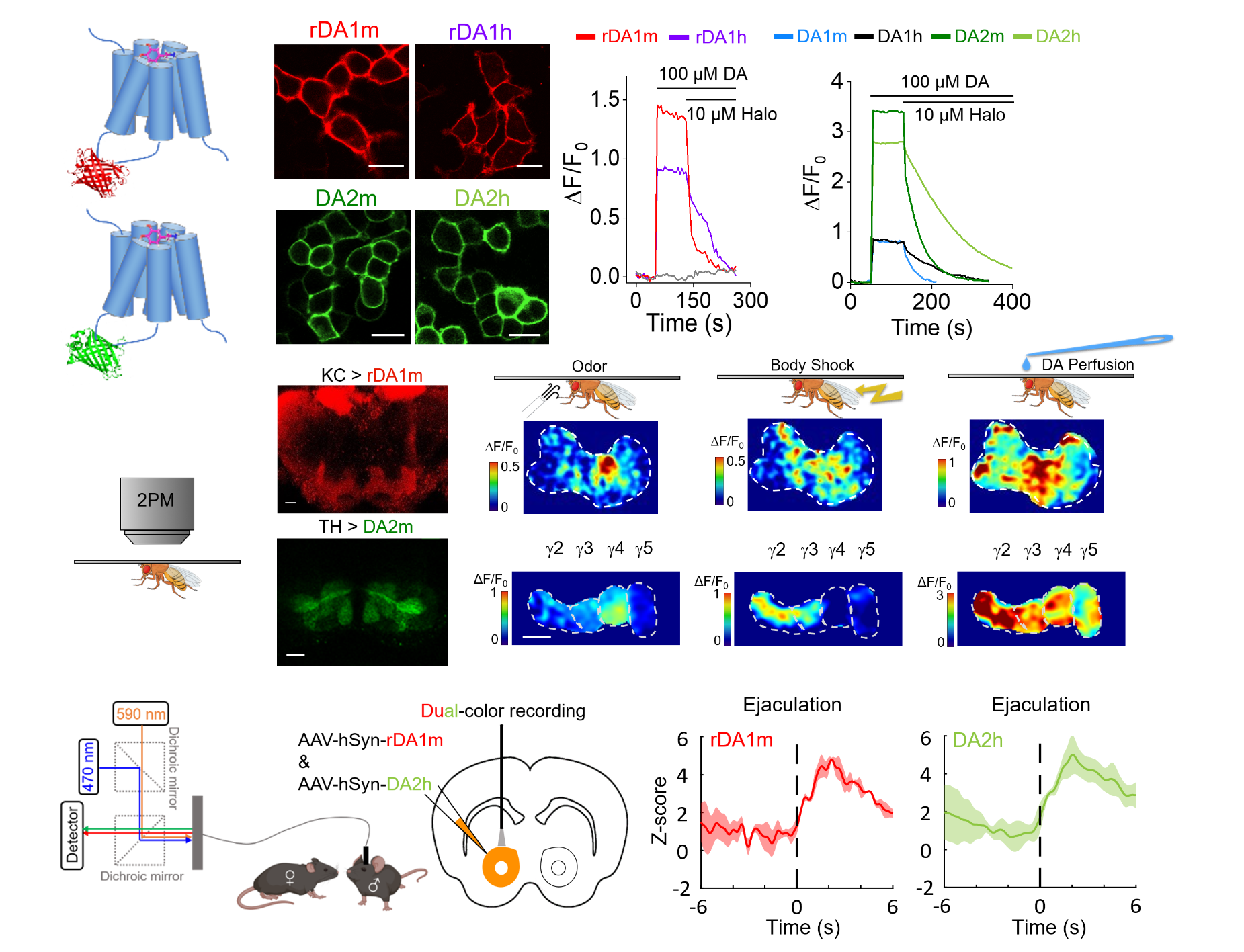 |
· Sun, F.#, Zhou, J.#, Dai, B.#, Qian, T., Zeng, J., Li, X., Zhuo, Y., Zhang, Y., Wang, Y., Qian, C., Tan, K., Feng, J., Dong, H., Lin, D.*, Cui, G.*, & Li, Y.*.(2020).
Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nature Methods. 17(11), 1156-1166.
[Full Text]
[PDF] |
|
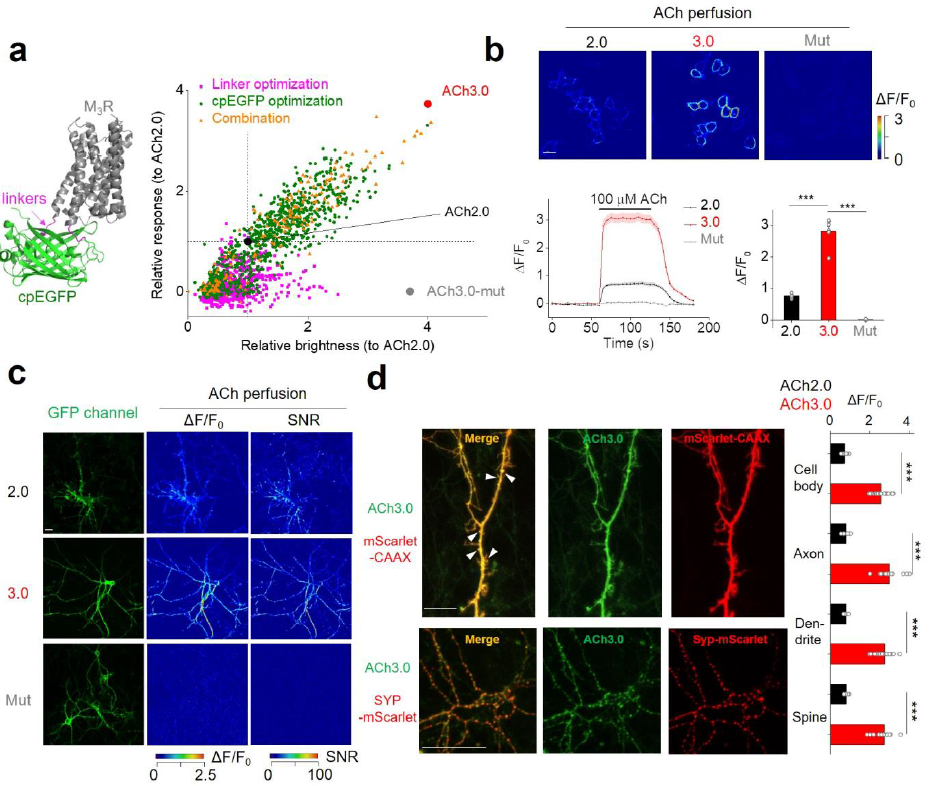 |
· Jing, M.*, Li, Y., Zeng, J., Huang, P., Skirzewski, M., Kljakic, O., Peng, W., Qian, T., Tan, K., Zou, J. , Trinh, S., Wu, R., Zhang, S., Pan, S., Hires, S., Xu, M., Li, H., Saksida, L. M., Prado, V. F., Bussey, T., Prado, M. A. M., Chen, L., Cheng, H., Li, Y.*.(2020).
An optimized acetylcholine sensor for monitoring in vivo cholinergic activity. Nature Methods, 17(11), 1139-1146.
[Full Text]
[PDF] |
|
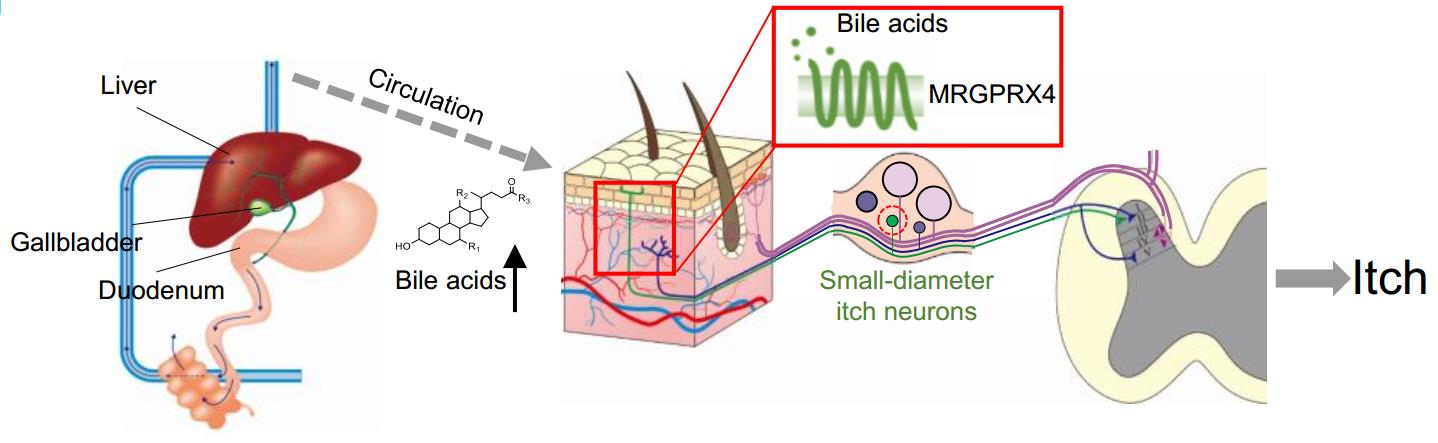 |
· Yu, H., Zhao, T., Liu, S., Wu, Q., Johnson, O., Wu, Z., Zhuang, Z., Shi, Y., He, R., Yang, Y., Sun, J., Wang, X., Xu, H., Zeng, Z., Lei, X., Luo, W.* & Li, Y.*.
(2019). MRGPRX4 is a bile acid receptor for human cholestatic itch. eLife, 8, e48431.
[Full Text]
[PDF] |
|
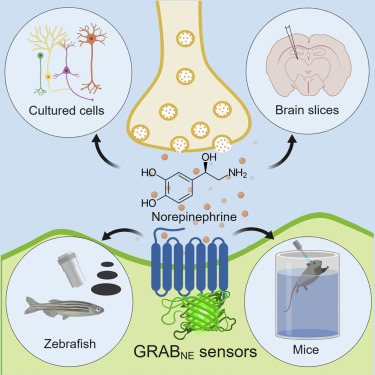 |
· Feng, J., Zhang, C., Lischinsky, J. E., Jing, M., Zhou, J., Wang, H., Zhang, Y., Dong, A., Wu, Z., Wu, H., Chen, W., Zhang, P., Zou, J., Hires, S. A., Zhu, J. J., Cui, G., Lin, D., Du, J. & Li, Y.* (2019). A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine. Neuron, 102(4), 745-761.
[Full Text]
[PDF] |
|
 |
· Wu, Z.#, Feng, J.#, Jing, M., & Li, Y.* (2019). G protein-assisted optimization of GPCR-activation based (GRAB) sensors.
Neural Imaging and Sensing 2019, vol. 10865, p. 108650N. International Society for Optics and Photonics.
[Full Text]
[PDF] |
|
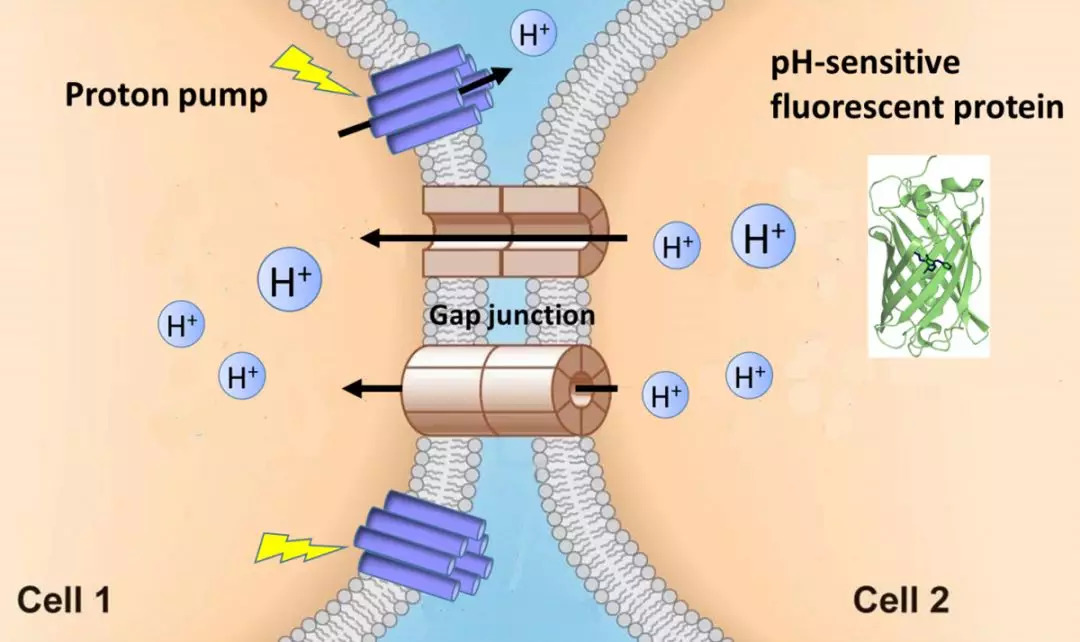 |
· Wu, L., Dong, A., Dong, L., Wang, S. Q., & Li, Y*. (2019). PARIS, an optogenetic method for functionally mapping gap junctions. eLife, 8, e43366.
[Full Text]
[PDF] * See Insight by: Kick, D. R., & Schulz, D. J. (2019). Cell Communication: Studying gap junctions with PARIS. eLife, 8, e45207. [Full Text][PDF] |
|
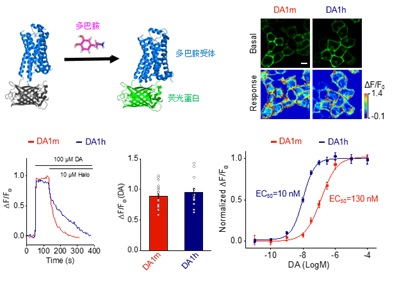 |
· Sun, F.#, Zeng, J.#, Jing, M.#, Zhou, J.,
Feng, J., Owen, S., Luo, Y., Li, F., Wang, H., Yamaguchi, T., Yong, Z.,
Gao, Y., Peng, W., Wang, L., Zhang, S., Du, J., Lin, D., Xu, M., Kreitzer, A. C., Cui, G.
& Li, Y.* (2018). A genetically-encoded fluorescent sensor enables rapid and
specific detection of dopamine in flies, fish, and mice. Cell,
174(2), 481-496. [Full Text] [PDF][Suppl Video 1][Suppl Video 2] * See Viewpoint by: Beyene, A. G., Delevich, K., Yang, S. J., & Landry, M. P. (2018). New optical probes bring dopamine to light. Biochemistry, 6379-6381. [Full Text][PDF> |
|
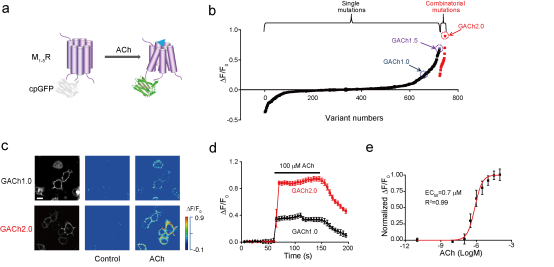 |
· Jing, M.#, Zhang, P.#, Wang, G., Feng, J., Mesik, L., Zeng, J., Jiang, H., Wang, S., Looby, J. C., Guagliardo, N. A., Langma, L. W., Lu, J., Zuo, Y., Talmage, D. A., Role, L. W., Barrett, P. Q., Zhang, L. I., Luo, M., Song, Y., Zhu, JJ* & Li, Y*.
(2018). A genetically-encoded fluorescent acetylcholine indicator for in vitro and in vivo studies. Nature Biotechnology, 36(8), 726-737.
[Full Text]
[PDF][Suppl Figs]
[Suppl Videos] * See Research Highlight by: Vogt, N. (2018). Detecting acetylcholine. Nature methods, 15(9), 648. [Full Text] [PDF] |
|
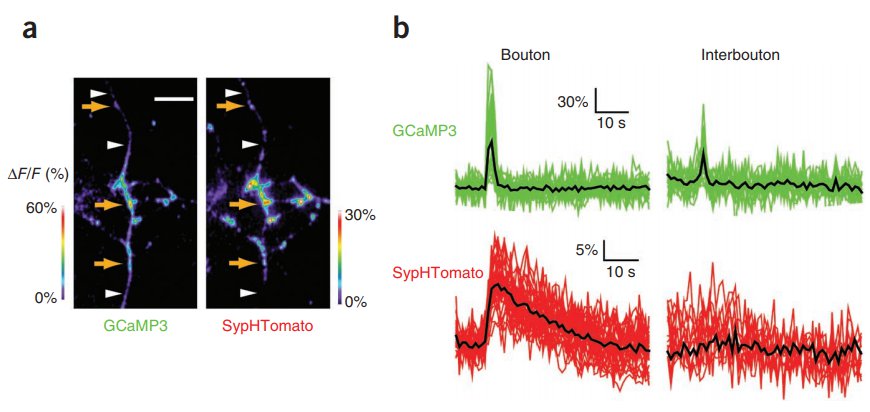 |
· Li, Y.*, & Tsien, R. W.* (2012). pHTomato, a red, genetically encoded indicator that enables multiplex interrogation of synaptic activity. Nature neuroscience, 15(7), 1047-1053.
[Full Text]
[PDF] |
|
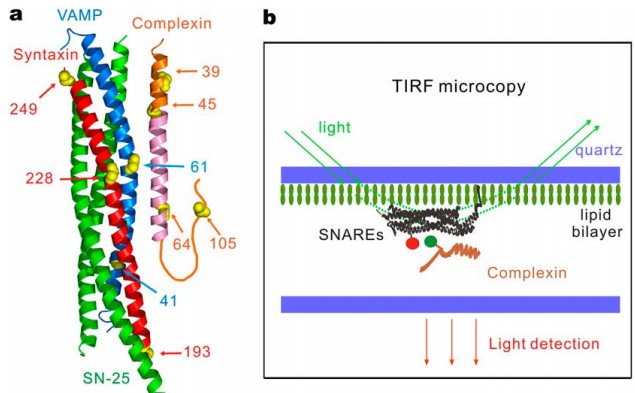 |
· Li, Y., Augustine, G. J., & Weninger, K.* (2007). Kinetics of complexin binding to the SNARE complex: correcting single molecule FRET measurements for hidden events. Biophysical journal, 93(6), 2178-2187. [Full Text] [PDF] |
Reviews, Book Chapters and Highlights
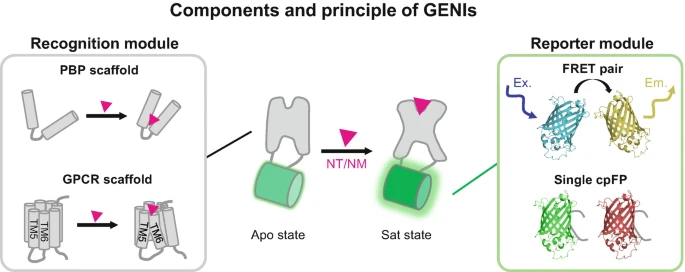 |
· Deng, F., Feng, J., Xie,H., & Li, Y.* (2025).
Mesoscopic Imaging of Neurotransmitters and Neuromodulators with Genetically Encoded Sensors.
Awake Behaving Mesoscopic Brain Imaging. Neuromethods, vol 214. Humana, New York.
[Full Text]
[PDF] |
|
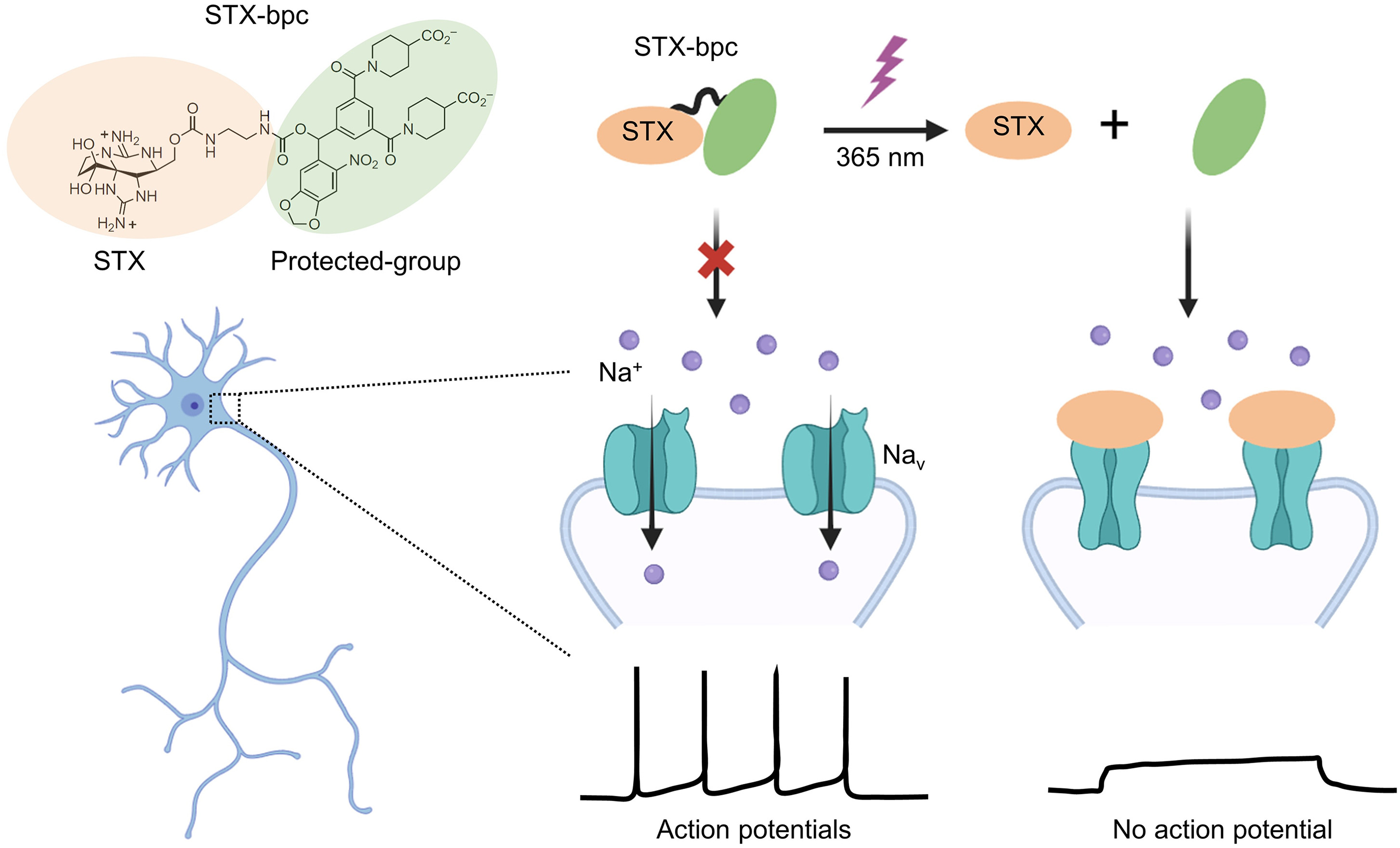 |
· Wan, J., & Li, Y.* (2024).
STX-bpc: “Brightening” the path to neuronal inhibition.
Cell Chemical Biology. 31(7): 1233-1235.
[Full Text]
[PDF] |
|
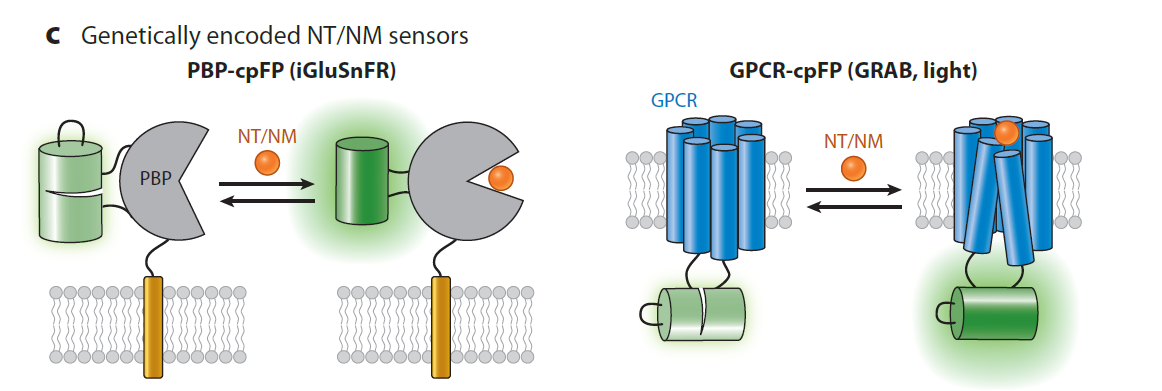 |
· Yang, Y.#, Li, B.#, & Li, Y.* (2024).
Genetically Encoded Sensors for the In Vivo Detection of Neurochemical Dynamics.
Annual Review of Analytical Chemistry. 17.
[Full Text]
[PDF] |
|
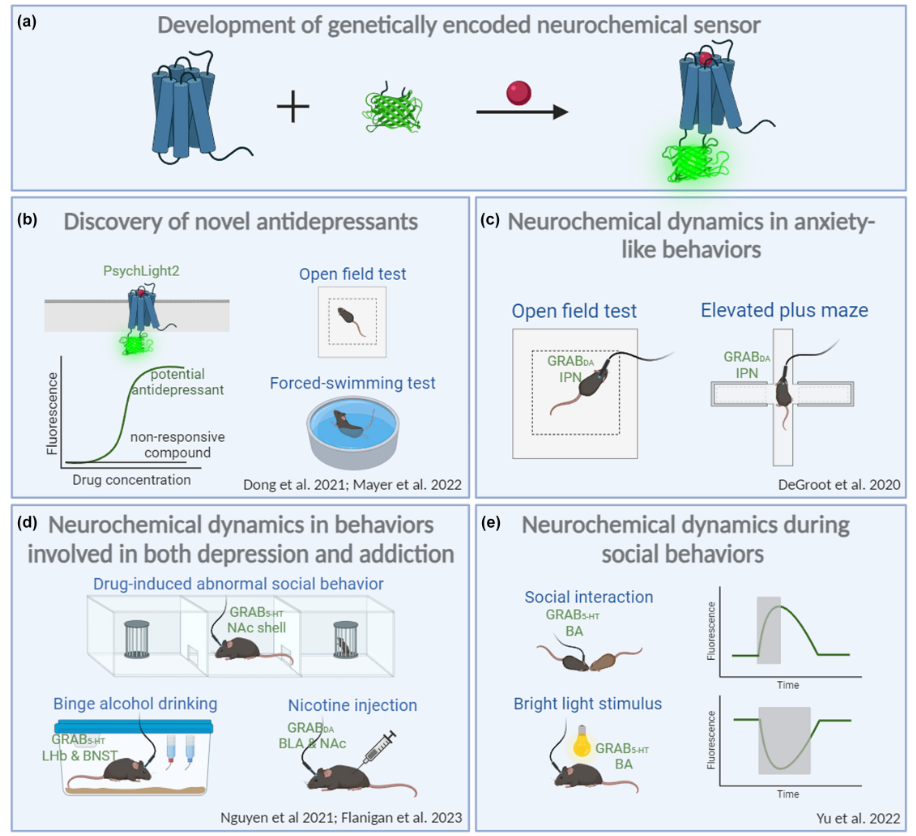 |
· Zhao, Y., Wan, J., & Li, Y.* (2024).
Genetically encoded sensors for in vivo detection of neurochemicals relevant to depression.
Journal of Neurochemistry. 17.
[Full Text]
[PDF] |
|
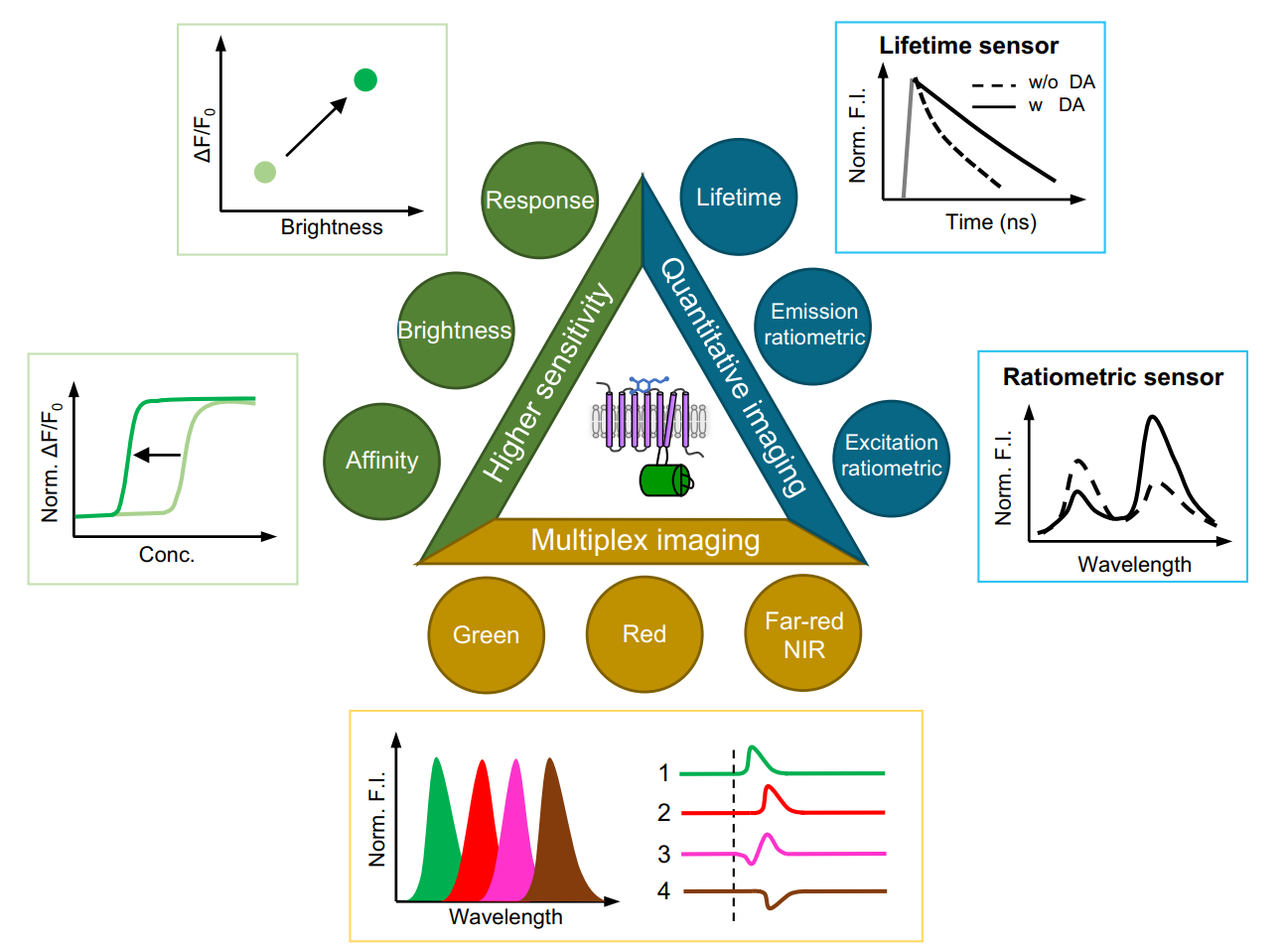 |
· Zheng, Y., & Li, Y.* (2023).
Past, Present, and Future of Tools for Dopamine Detection.
Neuroscience, 525, 13-25.
[Full Text]
[PDF] |
|
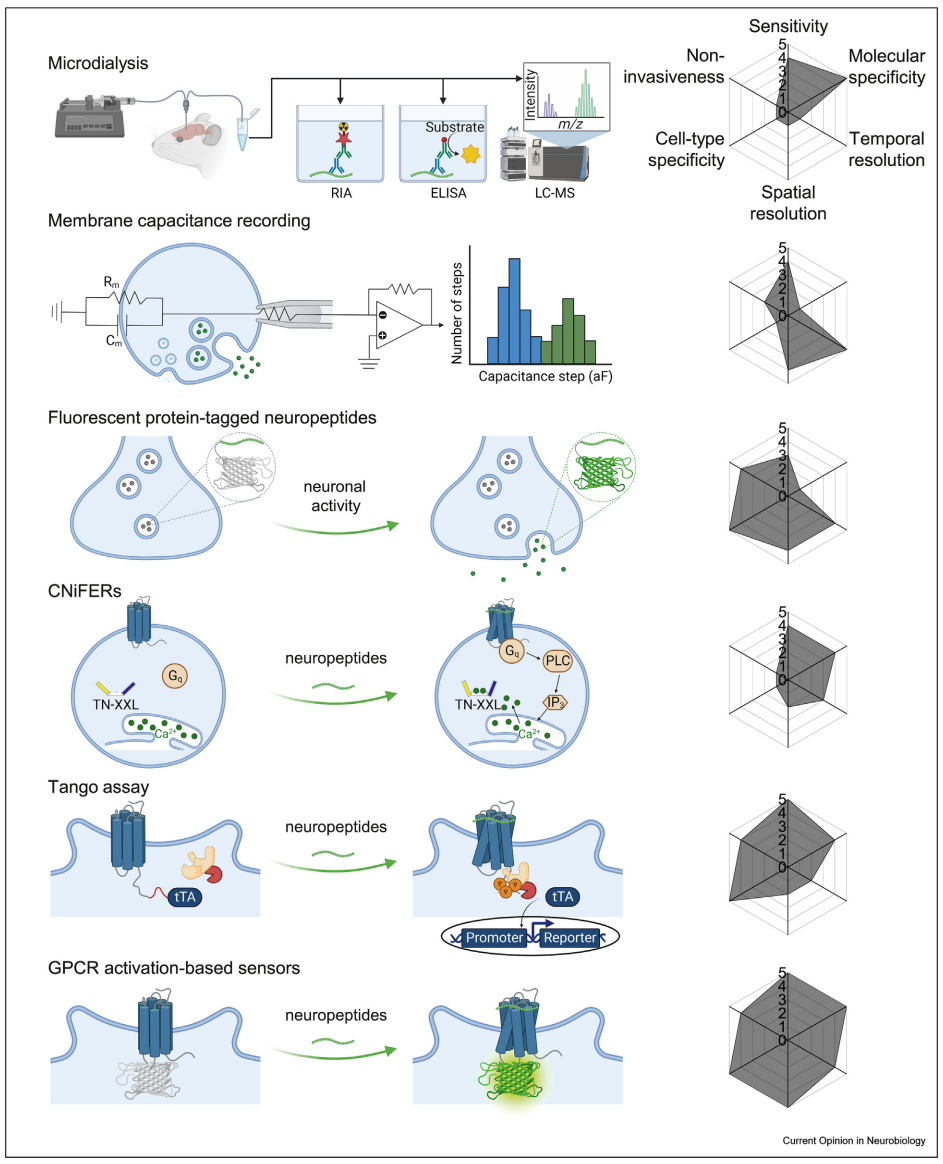 |
· Qian, T., Wang, H., Xia, X., & Li, Y.* (2023)
Current and emerging methods for probing neuropeptide transmission.
Current Opinion in Neurobiology, 81, 102751.
[Full Text]
[PDF] |
|
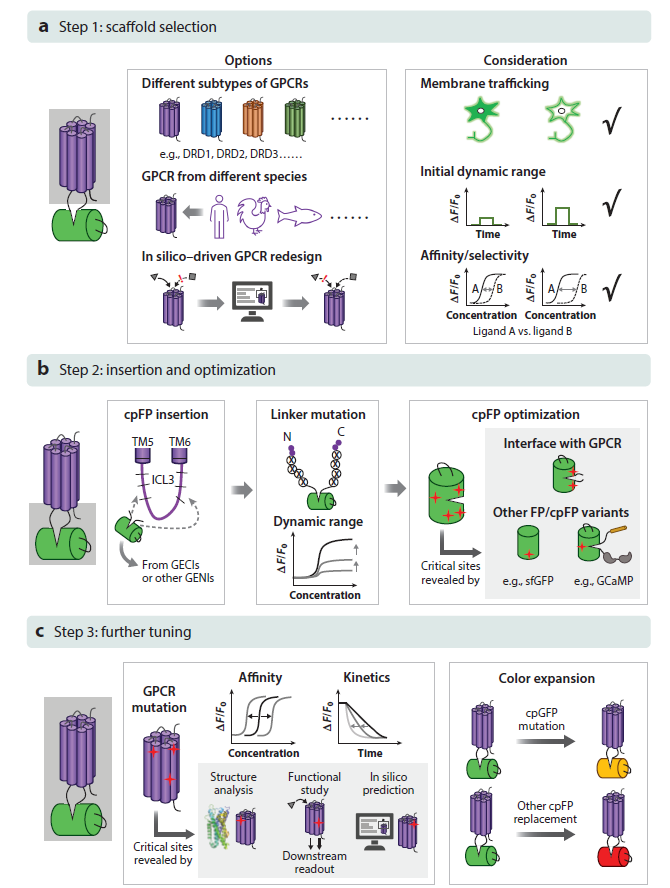 |
· Dong, C.#, Zheng, Y.#, Long-Iyer, K., Wright, E. C., Li, Y.*, & Tian, L.* (2022).
Fluorescence imaging of neural activity, neurochemical dynamics, and drug-specific receptor conformation with genetically encoded sensors.
Annual Review of Neuroscience. 45(1): 273-294.
[Full Text]
[PDF] |
|
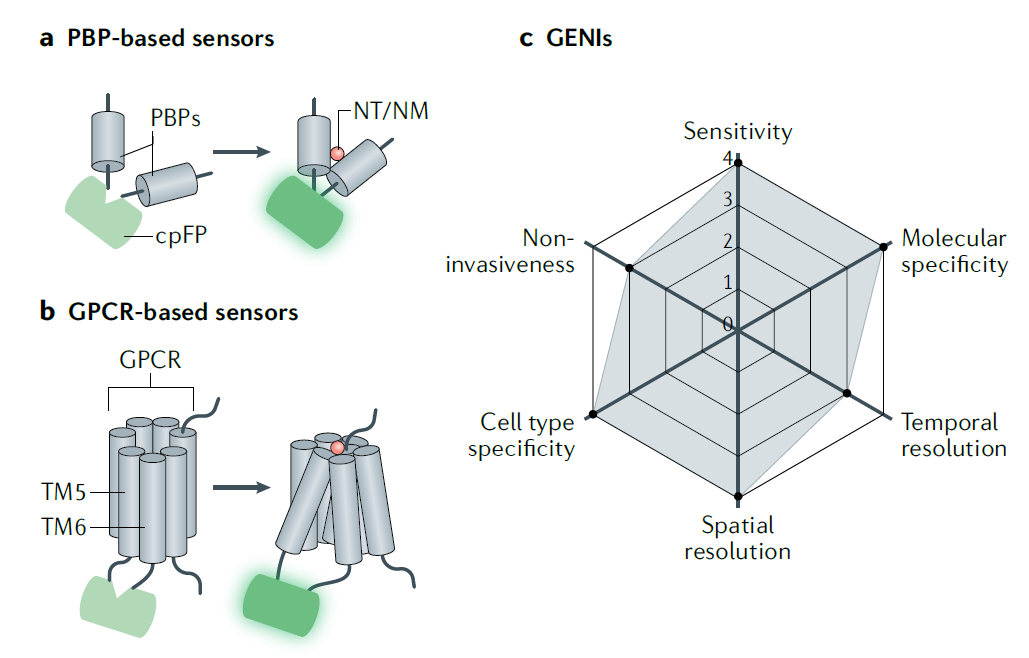 |
· Wu, Z., Lin, D., & Li, Y.* (2022).
Pushing the frontiers: tools for monitoring neurotransmitters and neuromodulators.
Nature Reviews Neuroscience. 23(5): 257-274.
[Full Text]
[PDF] |
|
 |
· Zhuo, Y., Li, Y.* (2022). New imaging methods for monitoring dopaminergic neurotransmission. Science China Life Sciences, 65.
[Full Text]
[PDF] |
|
 |
· Yulong Li. (2021). Neuron, 109(21), 3346-3348.
[Full Text]
[PDF] |
|
 |
· Yu, H., Wangensteen, K., Deng, T., Li, Y., & Luo, W.* (2021). MRGPRX4 in Cholestatic Pruritus. Semin Liver Dis41(03), 358-367.
[Full Text]
[PDF] |
|
 |
· Wan, J. & Li, Y.* (2020). Recent advances in detection methods for neurotransmitters. Chinese Journal of Analytical Chemistry, 48(3), 307-315. (In Chinese)
[Full Text]
[PDF] |
|
 |
· Wu, Z.* & Li, Y.* (2020). New frontiers in probing the dynamics of purinergic transmitters in vivo. Neuroscience Research, https://doi.org/10.1016/j.neures.2020.01.008.
[Full Text]
[PDF] |
|
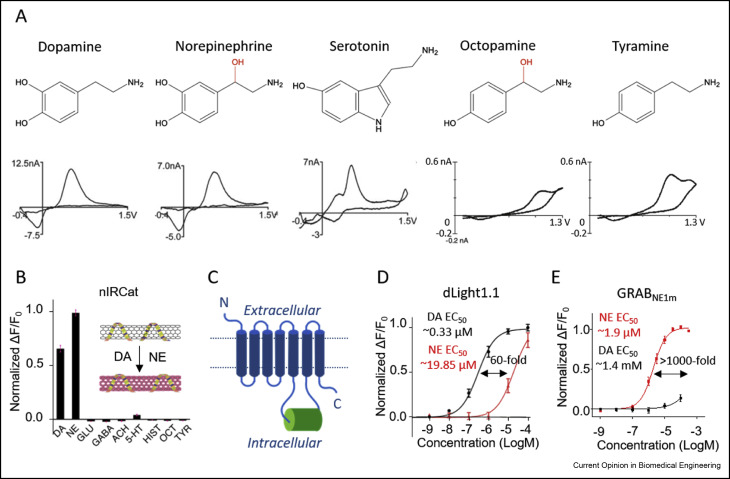 |
· Zeng, J., Sun, F., Wan, J., Feng, J. & Li, Y.* (2019). New optical methods for detecting monoamine neuromodulators. Current Opinion in Biomedical Engineering, https://doi.org/10.1016/j.cobme.2019.09.010.
[Full Text]
[PDF] |
|
 |
· Jing, M., Zhang, Y., Wang, H. & Li, Y.* (2019). GPCR‐based sensors for imaging neurochemicals with high sensitivity and specificity. Journal of Neurochemistry, https://doi.org/10.1111/jnc.14855. [Full Text] [PDF] |
|
 |
· Dong, A.*, Liu, S., & Li, Y.* (2018). Gap junctions in the nervous system: probing functional connections using new imaging approaches. Frontiers in Cellular Neuroscience, 12, 320.
[Full Text]
[PDF] |
|
 |
· Wang, H., Jing, M., & Li, Y.* (2018). Lighting up the brain: genetically encoded fluorescent sensors for imaging neurotransmitters and neuromodulators. Current Opinion in Neurobiology, 50, 171-178.
[Full Text]
[PDF] |
|
 |
· Wang, A.#, Feng, J.#, Li, Y.*, & Zou, P.* (2018). Beyond fluorescent proteins: hybrid and bioluminescent indicators for imaging neural activities. ACS chemical neuroscience, 9(4), 639-650.
[Full Text]
[PDF] |
|
 |
· Qian, C., & Li, Y.* (2015). Spine maturation and pruning during development: Cadherin/Catenin complexes come to help. Science China. Life sciences,58(9), 929.
[Full Text]
[PDF] |
|
 |
· Li, Y.*, & Rao, Y.* (2015). Pied piper of neuroscience. Cell, 163(2), 267-268.
[Full Text]
[PDF] |
Pre-prints
 |
· Lopez, R., Noble, N., Ozcete, O., Cai, X., Handy, G., Andersen, J., Patriarchi, T., Li, Y*, & Kaeser, P.* (2024).
Innervation density governs crosstalk of GPCR-based norepinephrine and dopamine sensors.
bioRxiv.
[Full Text]
[PDF] |
|
 |
· Xia, X., & Li, Y*. (2024).
A new GRAB sensor reveals differences in the dynamics and molecular regulation between neuropeptide and neurotransmitter release.
bioRxiv, 2024.2005.2022.595424.
[Full Text]
[PDF] |
|
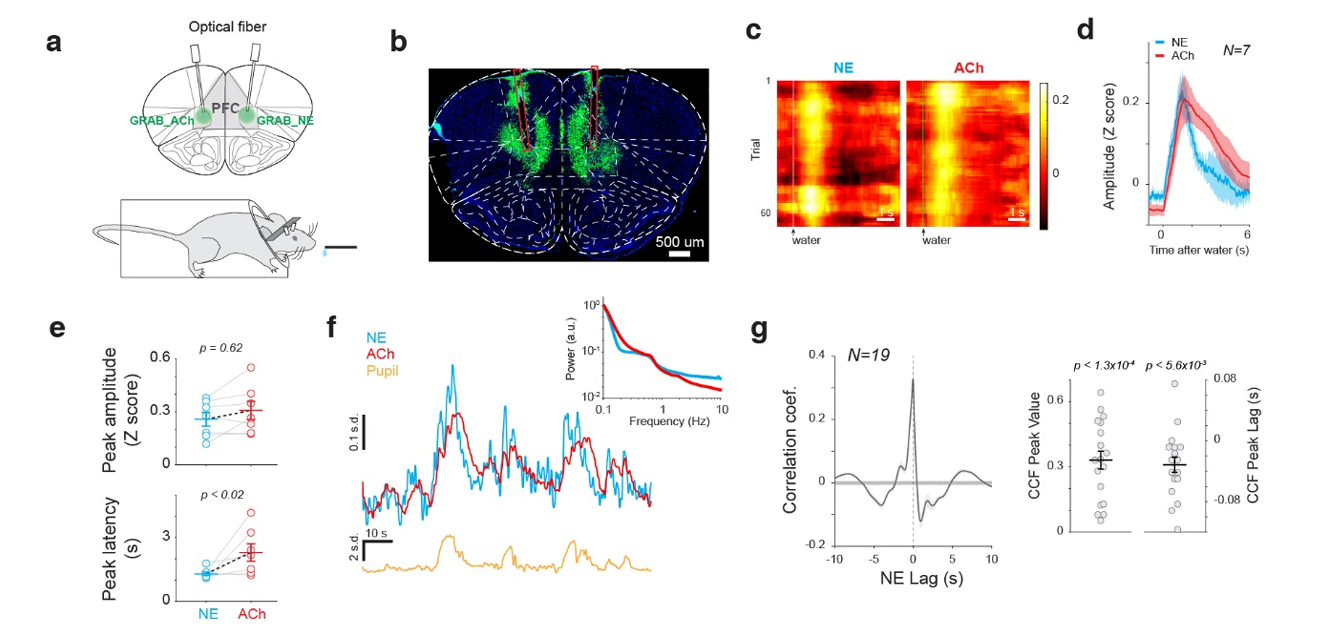 |
· Liu, Y., Nong, Y., Feng, J., Li, G., Sajda, P., Li, Y., & Wang, Q.* (2024).
Phase synchrony between prefrontal noradrenergic and cholinergic signals indexes inhibitory control.
bioRxiv, 2022.2005.2018.492553.
[Full Text]
[PDF] |
|
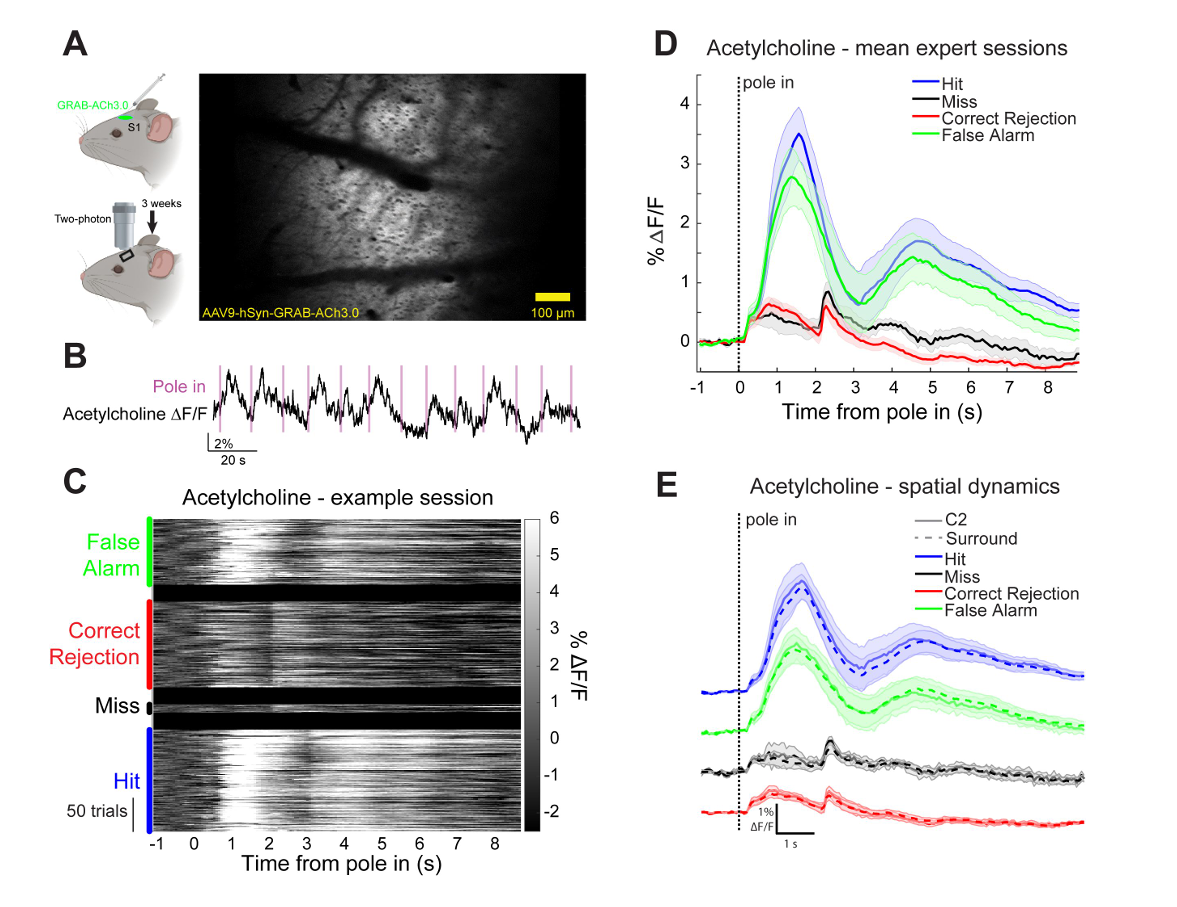 |
· Zou, J., Willem, J., Mridha, Z., Trinh, S., Erskine, A., Jing, M., Yao, J., Walker, S., Li, Y., McGinley, M., Hires, S.* (2024)
Goal-directed motor actions drive acetylcholine dynamics in sensory cortex
eLife , 13:RP96931
[Full Text]
[PDF] |
|
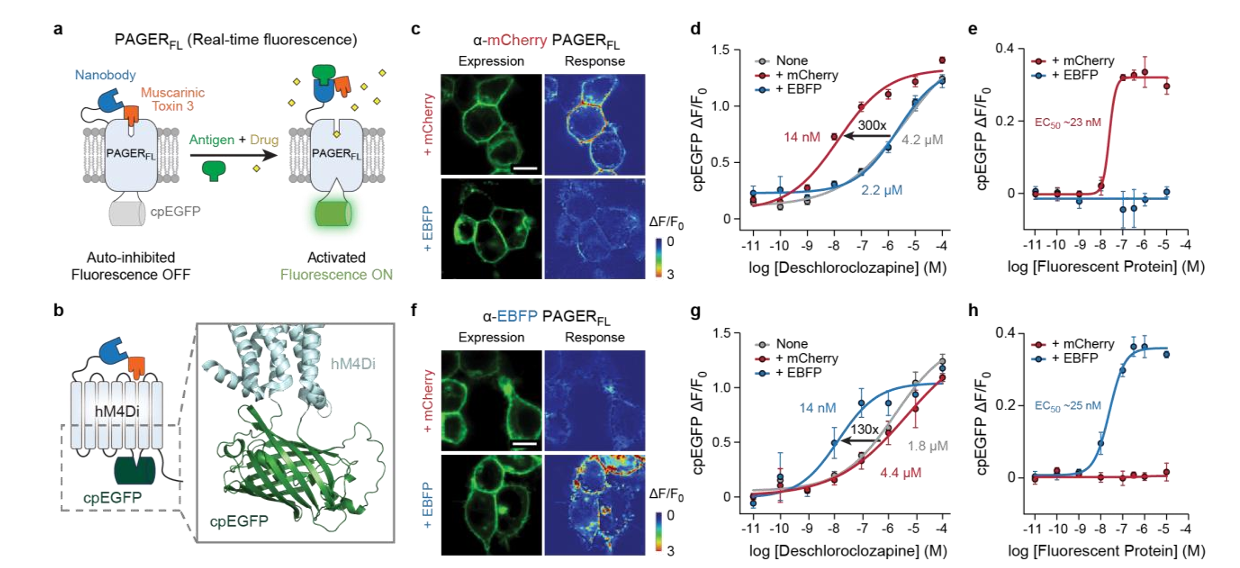 |
· Costa, K. M.#*, Zhang, Z.#*, Zhuo, Y., Li, G., Li, Y., & Schoenbaum, G.* (2024).
Dopamine and acetylcholine correlations in the nucleus accumbens depend on behavioral task states.
bioRxiv, 2024.2005.2003.592439.
[Full Text]
[PDF] |
|
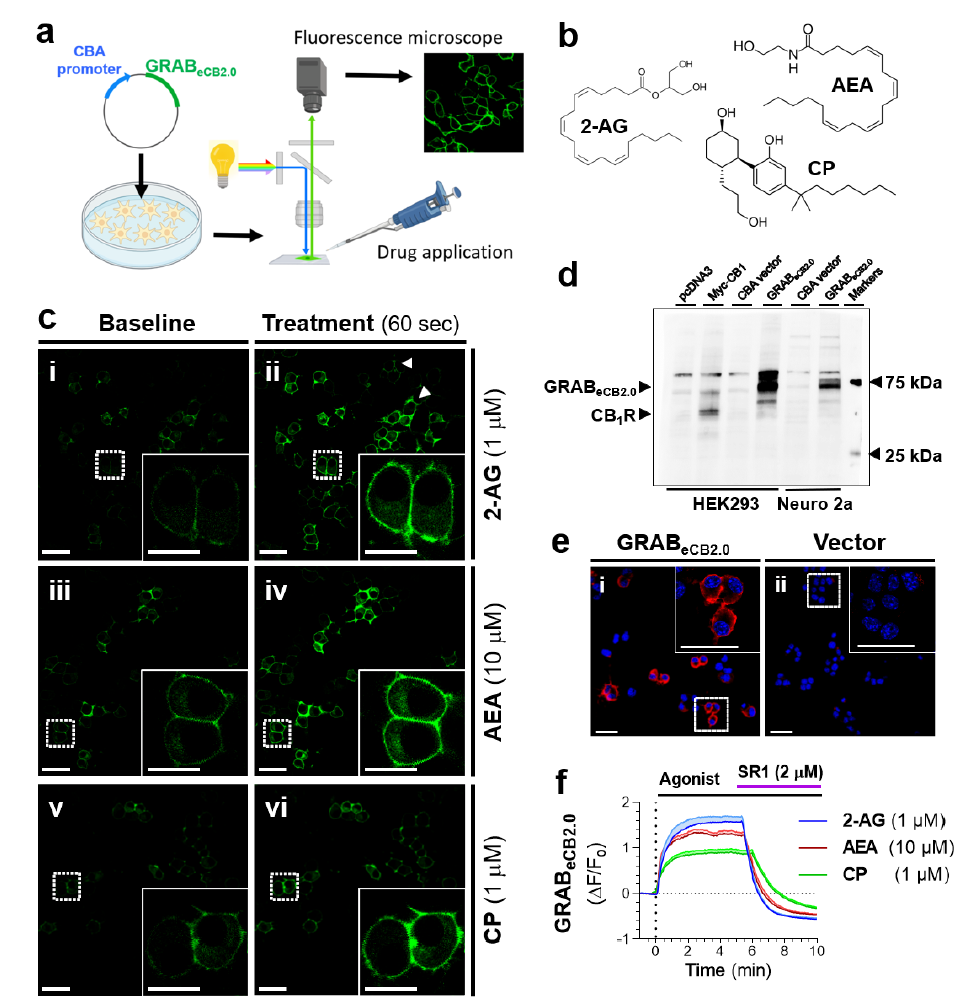 |
· Kalogriopoulos, N. A.#, Tei, R.#, Yan, Y., Ravalin, M., Li, Y., & Ting, A.* (2024).
Synthetic G protein-coupled receptors for programmable sensing and control of cell behavior.
bioRxiv, 2024.2004.2015.589622.
[Full Text]
[PDF] |
|
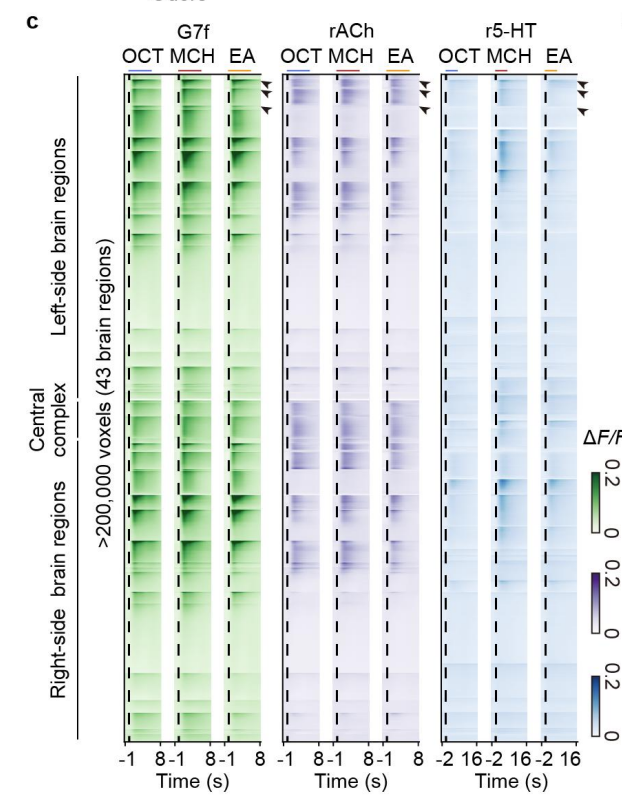 |
· Fan, J., Wang, Y., Li, L., He, J., Zhao, Z., Deng, F., Li, G, Li X., Zhou, Y., Zhao, J., Li, Y., Wu, J., Fang, L., & Dai, Q* (2024).
Prominent involvement of acetylcholine in shaping stable olfactory representation across the Drosophila brain.
bioRxiv, 2024.04.03.587915.
[Full Text]
[PDF] |
|
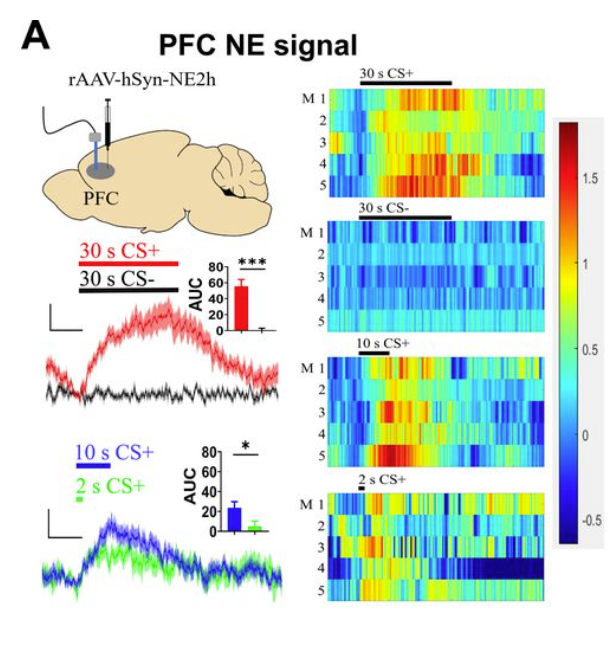 |
· Wang T., Zhang X., Duan H., Xia D., Li T., Yan R., Zhan Y., Li, Y., Gao W., & Zhou, Q.* (2024).
Gating of Memory to Behavior by the Locus Coeruleus.
bioRxiv, 2024.01.09.574947.
[Full Text]
[PDF] |
|
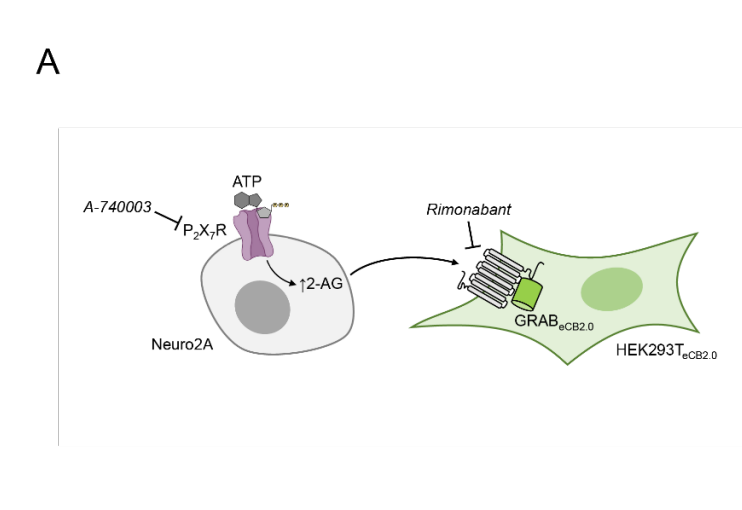 |
· Straub, V., Barti, B., Tandar, S., Stevens, A., van der Wel, T., Zhu, N., Rüegger, J., van der Horst, C., Heitman, L., Li, Y., Stella, N., van Hasselt, J. G., Coen Katona, I., van der Stelt, M.* (2024).
The endocannabinoid 2-arachidonoylglycerol is released and transported on demand via extracellular microvesicles.
bioRxiv, 2024.09.23.614520.
[Full Text]
[PDF] |
|
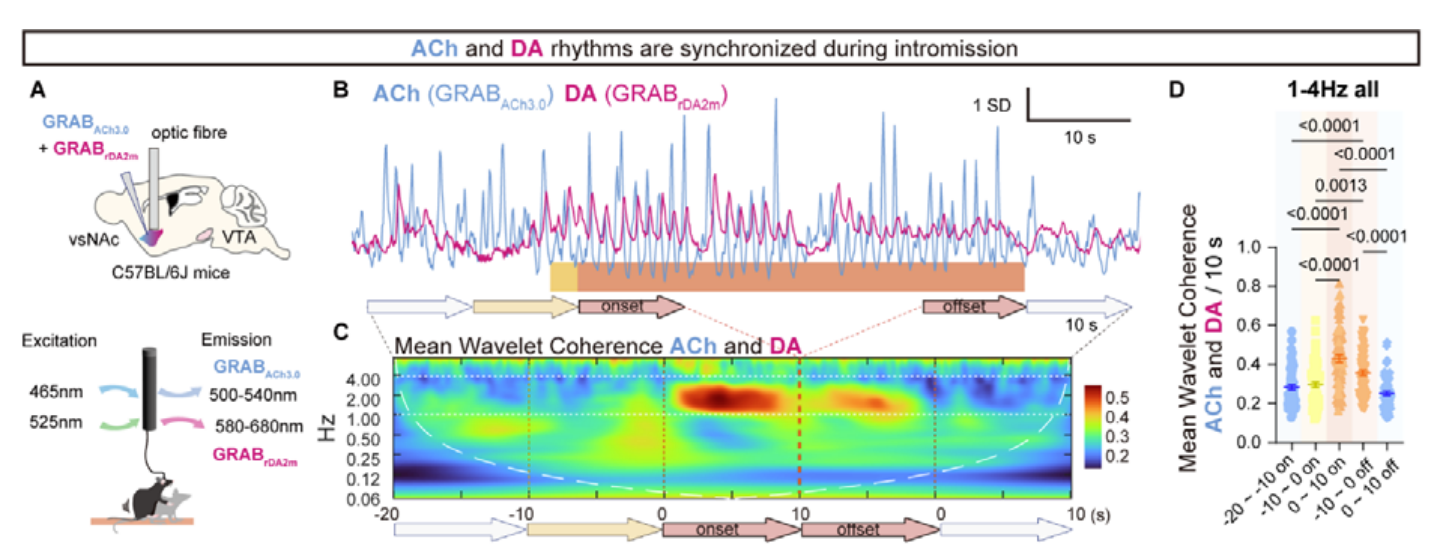 |
· Ai M., Takeshi K., Naoki N., Yuka T., Yoan C., Yukiko I., Li, Y., Hotaka T., Jun S., Masashi Y., Takeshi S., Katsuyasu S., & Liu, Q.* (2024).
Sequential Transitions of Male Sexual Behaviors Driven by Dual Acetylcholine-Dopamine Dynamics.
bioRxiv, 2023.12.21.572798.
[Full Text]
[PDF] |
|
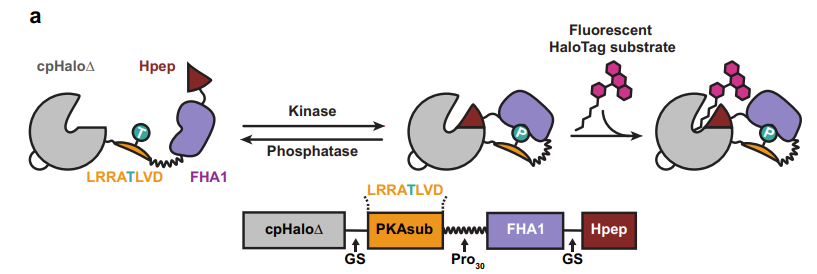 |
· Sun, D., Ng, S., Zheng, Y., Xie, S. , Schwan, N., Breuer P., Hoffmann, D., Michel, J., Azorin, D., Boonekamp, K., Winkler, F., Wick, W., Boutros, M., Li, Y., & Johnsson, K.* (2024).
Molecular recording of cellular protein kinase activity with chemical labeling.
bioRxiv, 2024.09.11.611894.
[Full Text]
[PDF] |
|
 |
· Ge, C., Chen, Z., Sun, F., Hou, R., Fan, H., Li, Y., & Li, C.* (2024).
Timing-dependent modulation of working memory by VTA dopamine release in medial prefrontal cortex.
bioRxiv, 2024.09.11.611894.
[Full Text]
[PDF] |
|
 |
· Wu, Y., Gu, X., Kong, Y., Yang, S., Wang, H., Xu, M., Wang, Q., Yi, X., Lin, Z., Jiao, Z., Cheung, H., Zhao, X., Bian, X., Jiang, Q., Li, Y., Zhu, M., Wang, L., Li, Y., Huang, J., Li, Q., Li, W., & Xu, T.* (2024).
Neuropeptide Y co-opts neuronal ensembles for memory lability and stability.
bioRxiv, 2024.05.09.593455.
[Full Text]
[PDF] |
|
 |
· Wang, H., Ortega, H., Kelly, E., Indajang, E., Feng, J. , Li, Y., & Kwan, A.* (2024).
Frontal noradrenergic and cholinergic transients exhibit distinct spatiotemporal dynamics during competitive decision-making.
bioRxiv, 2024.01.23.576893.
[Full Text]
[PDF] |
|
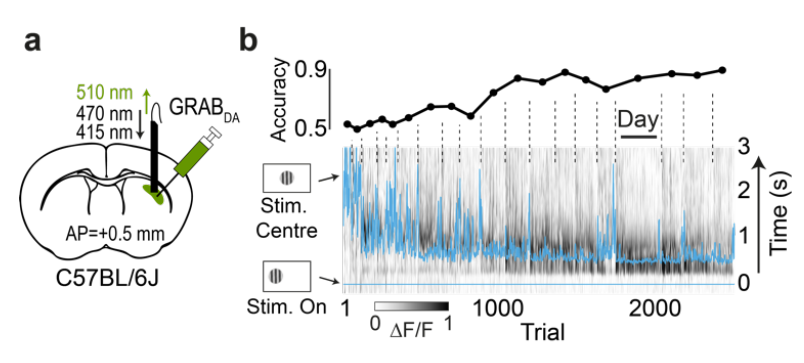 |
· Garcia, S., Laffere, A., Toschi, C., Schilling, L., Podlaski, J., Fritsche, M., Zatka-Haas, P., Li, Y., Bogacz, R., Saxe, A., & Lak, A.* (2023).
Striatal dopamine reflects individual long-term learning trajectories.
bioRxiv, 2023.12.14.571653.
[Full Text]
[PDF] |
|
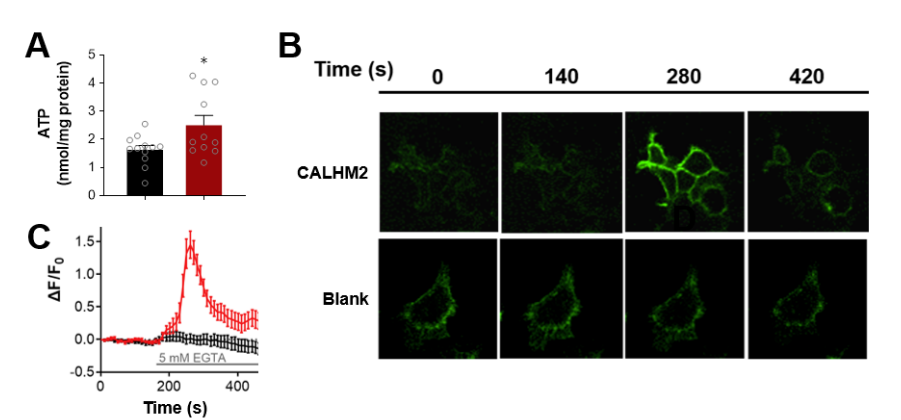 |
· Guo, Q., Hou, T., Xie, W., Zhang, J., Ma, X., Guo, Y., Wang, X., Wang, L., Lu, M., Wu, Z, Wang, H., Chen, Y., Li, Y., & Wang, S.*(2024).
Calcium Homeostasis Modulator 2 Constitutes an ATP-regulation Pore in Mitochondria.
bioRxiv, 2024.09.30.615983.
[Full Text]
[PDF] |
|
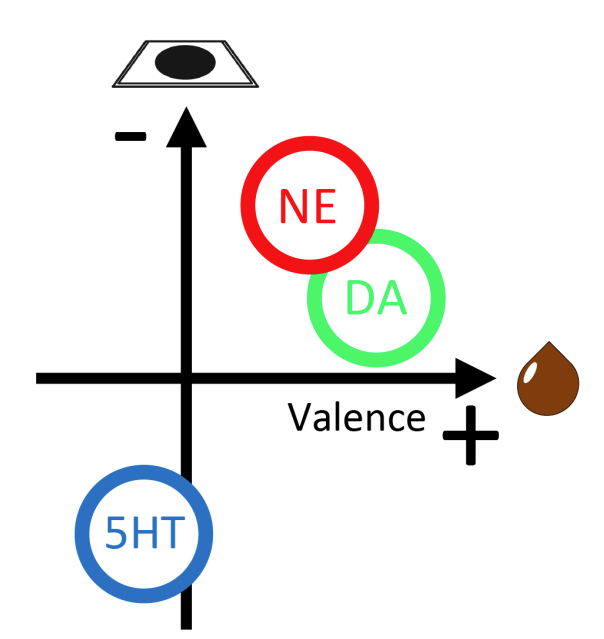 |
· Yang, J., Basu, A., Liu, R., Staszko, S., Yu, A., Rondeau, J., Glaeser-Khan, S., Feng, J., Li, Y., Che, A., & Kaye, A.* (2023).
Frontal cortex norepinephrine, serotonin, and dopamine dynamics in an innate fear-reward behavioral model.
bioRxiv, 2023.11.27.568929.
[Full Text]
[PDF] |
|
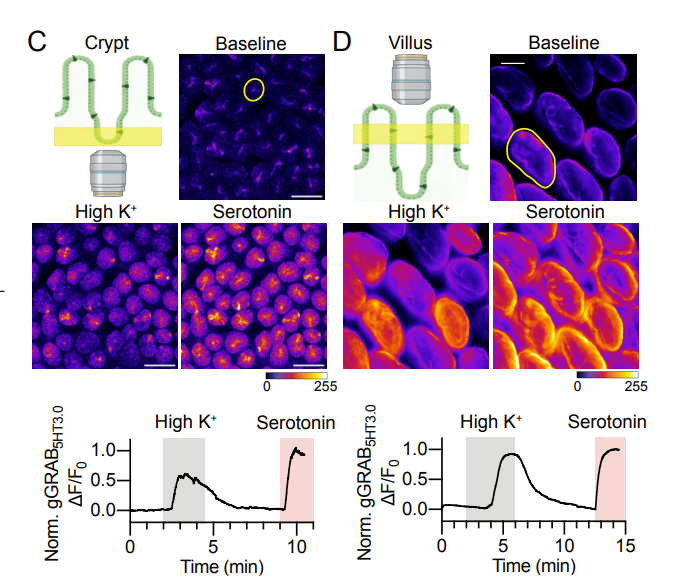 |
· Touhara, K., Rossen, N., Deng, F., Chu, T., Harrington, A., Caraballo, S., Brizuela, M., O'Donnell, T., Cil, O., Brierley, S., Li, Y., & Julius, D.* (2024).
Crypt and Villus Enterochromaffin Cells are Distinct Stress Sensors in the Gut.
bioRxiv, 2024.02.06.579180.
[Full Text]
[PDF] |
|
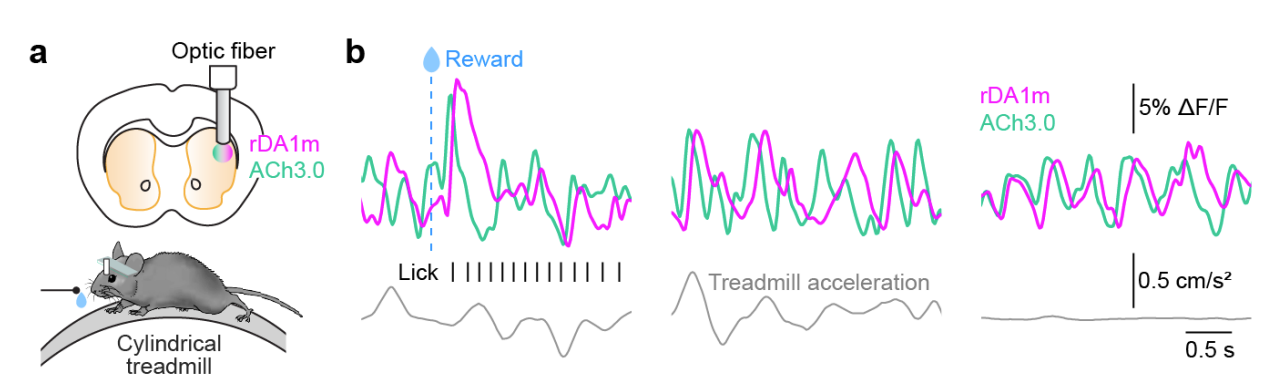 |
· Krok, A., Mistry, P., Li, Y., & Tritsch, N.* (2022).
Intrinsic reward-like dopamine and acetylcholine dynamics in striatum.
bioRxiv, 2022.09.09.507300.
[Full Text]
[PDF] |
|
 |
· Singh, S., Sarroza, D., English, A., Whittington, D., Dong, A., van der Stelt, M., Li, Y., Zweifel, L., Bruchas, M. R., Land, B. B., & Stella, N.* (2022).
ABHD6 selectively controls metabotropic-dependent increases in 2-AG production.
bioRxiv, 2024.2005.2017.594562.
[Full Text]
[PDF] |
|
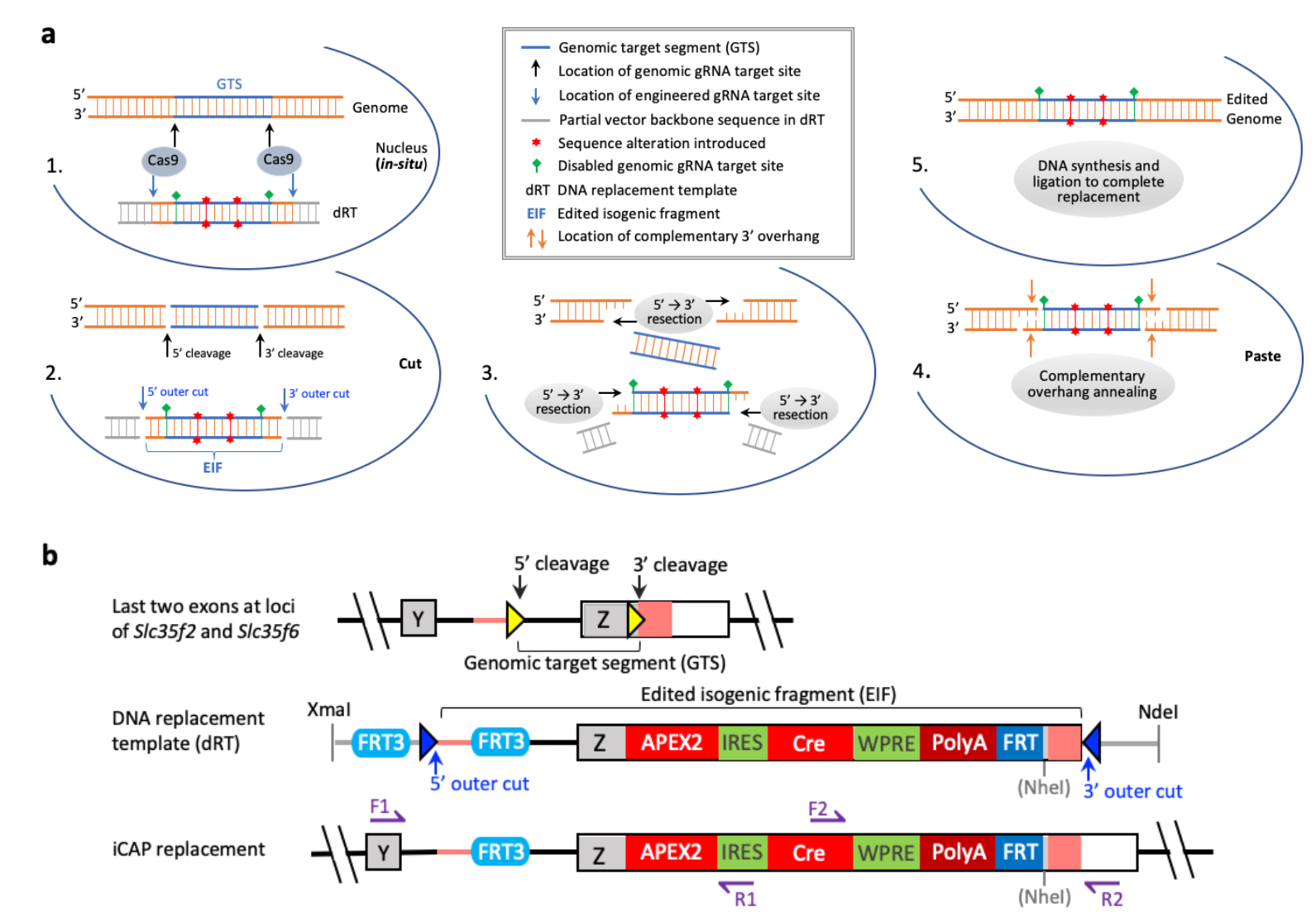 |
· Jiang, P.*, Kemper, K. M., Chang, K.-T., Qian, C., Li, Y., Guan, L., van Hasselt, P., Caradonna, S. J., & Strich, R. (2022).
An in situ cut-and-paste genome editing platform mediated by CRISPR/Cas9 or Cas12a.
bioRxiv, 2022.2003.2030.486486.
[Full Text]
[PDF] |
|
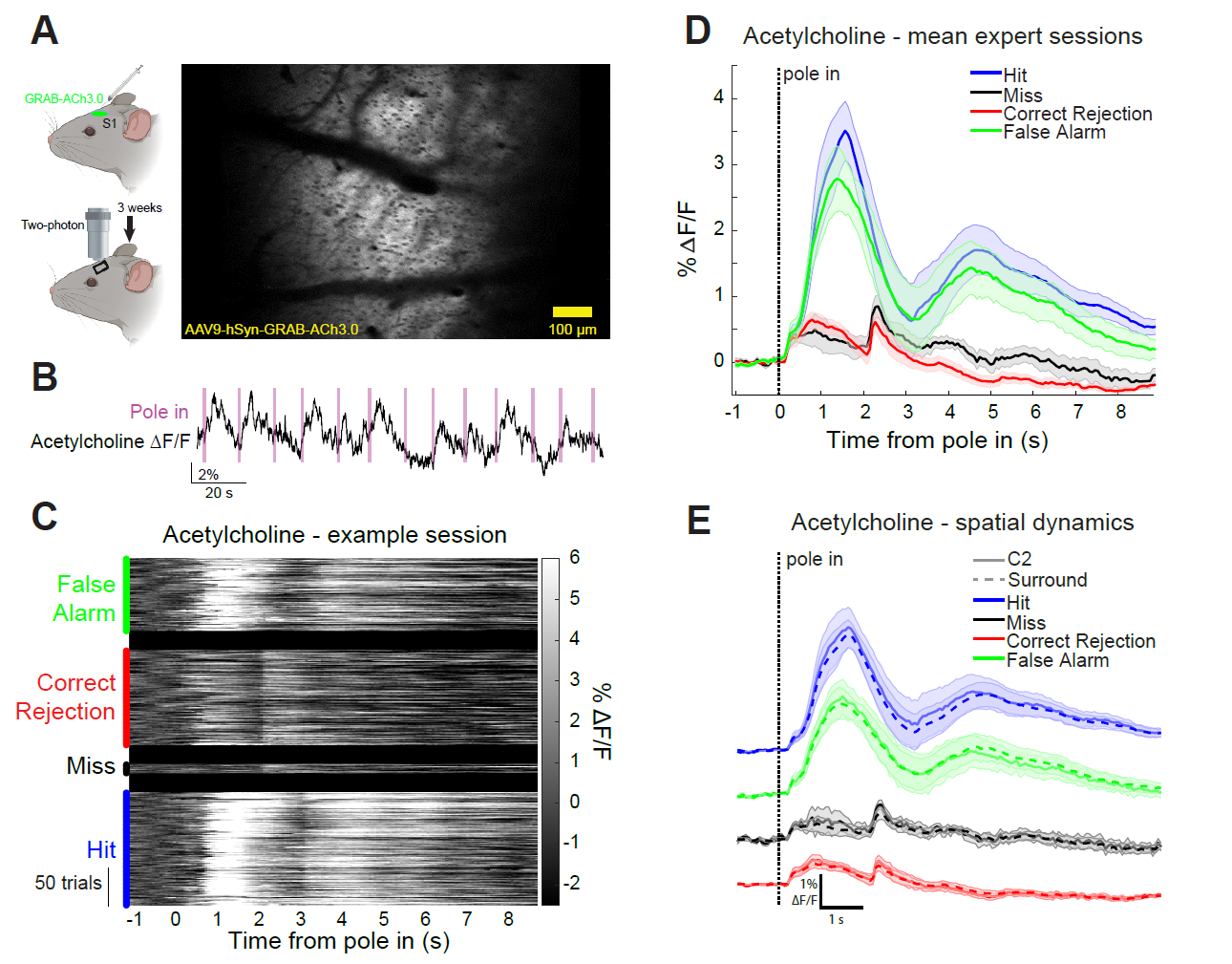 |
· Zou, J., Trinh, S., Erskine, A., Jing, M., Yao, J., Walker, S., Li, Y.., & Hires, S. A.* (2021). Directed motor actions and choice signalling drive cortical acetylcholine dynamics. bioRxiv,, 2021.2012.2021.473699.
[Full Text]
[PDF] |
|
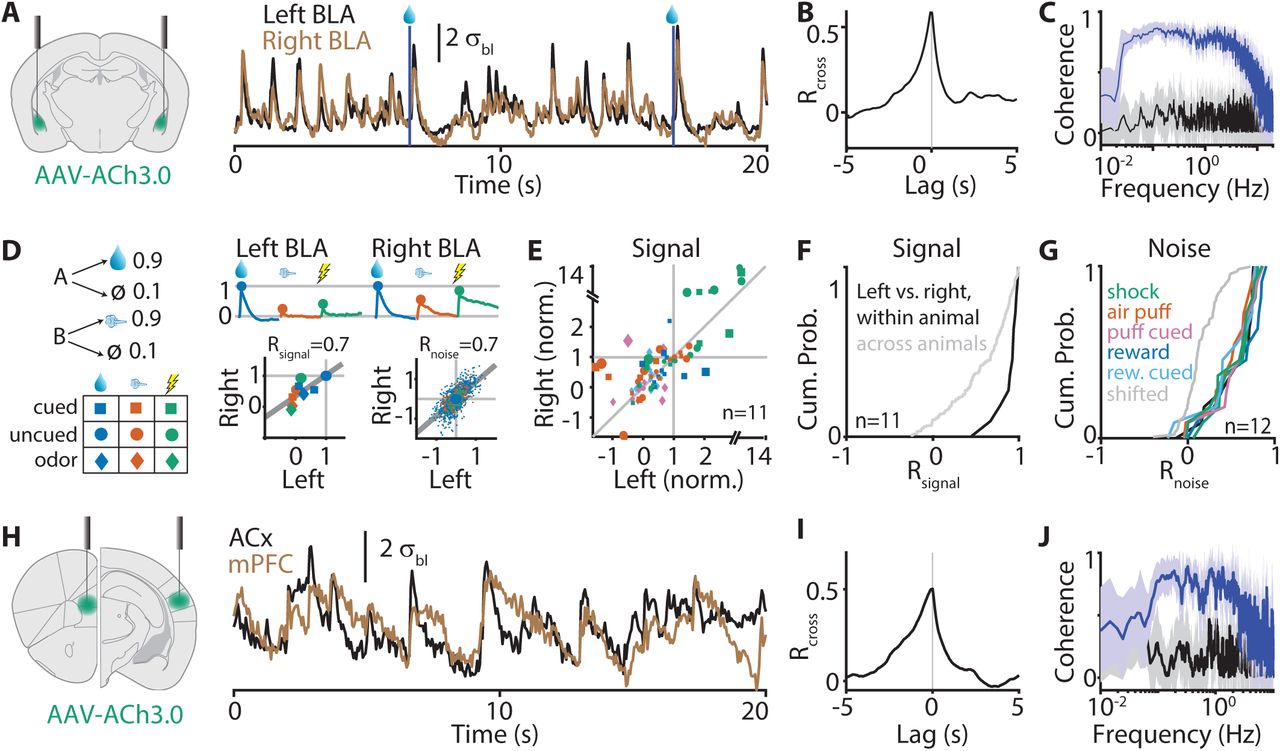 |
· Sturgill, J. F., Hegedus, P., Li, S. J., Chevy, Q, Siebels, A., Jing, M., Li, Y., Hangya, B.* & Kepecs, A.*(2020). Basal forebrain-derived acetylcholine encodes valence-free reinforcement prediction error. bioRxiv, 2020.02.17.953141.
[Full Text]
[PDF] |
Collaborative Publication
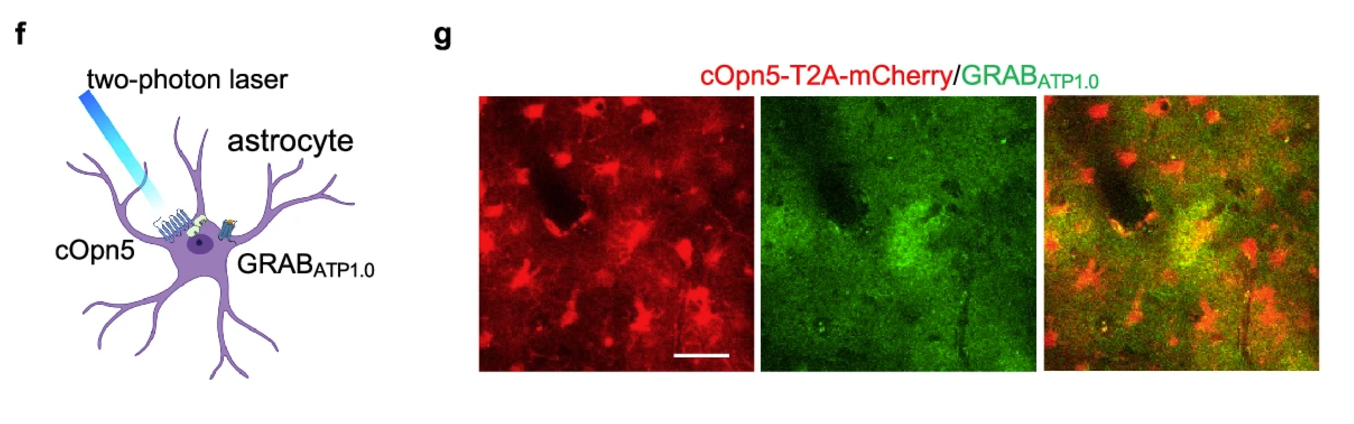 |
· Li, H., Zhao, Y., Dai, R. , Geng, P., Weng, D., Wu, W., Yu, F., Lin, R., Wu, Z. , Li, Y., & Luo, M.* (2024)
Astrocytes release ATP/ADP and glutamate in flashes via vesicular exocytosis.
Molecular Psychiatry.
[Full Text]
[PDF] |
|
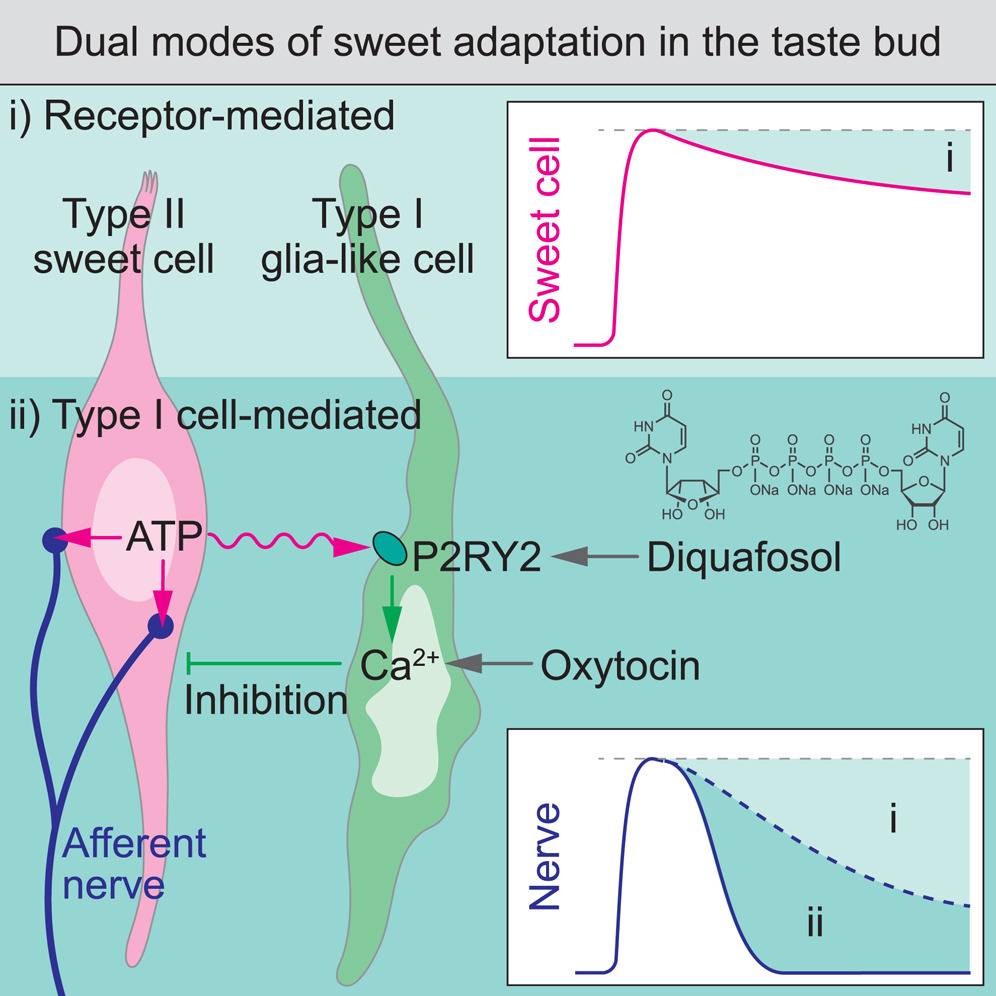 |
· Park, G., Lee, G., Yoon, J., Han, J., Choi, P., Kim, M., Lee, S., Park, C., Wu, Z. , Li, Y., & Choi, M.* (2024)
Glia-like taste cells mediate an intercellular mode of peripheral sweet adaptation.
Cell.
[Full Text]
[PDF] |
|
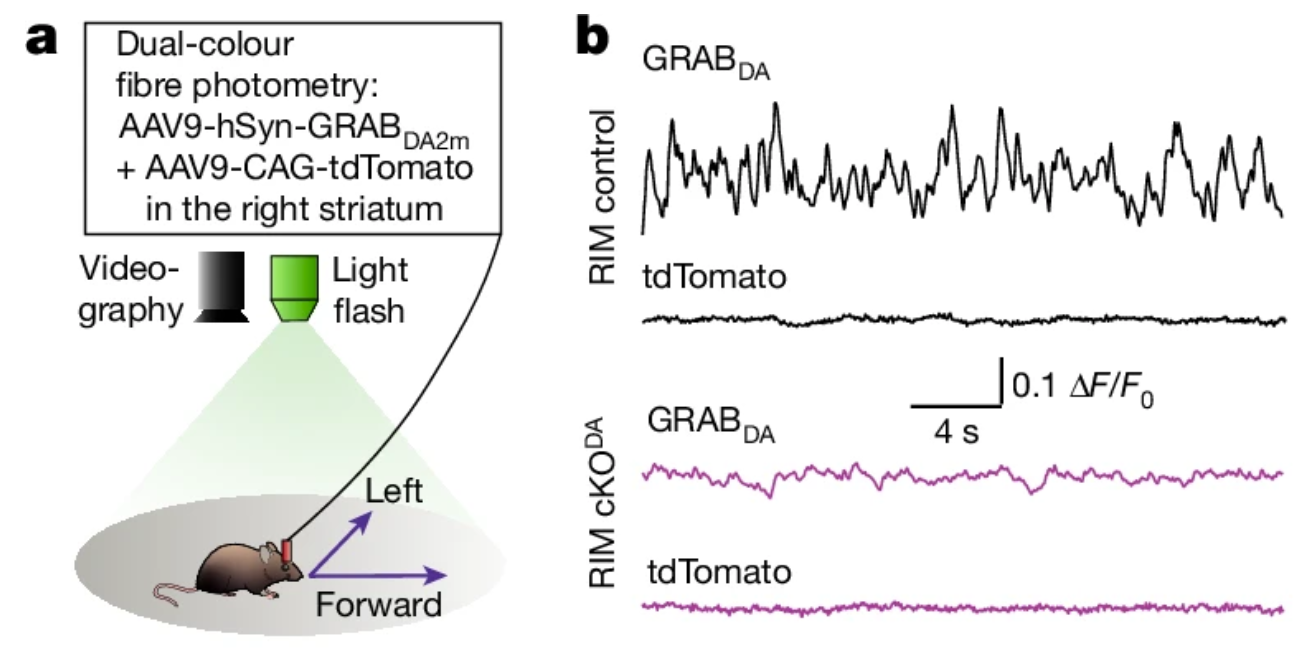 |
· Cai, X., Liu, C., Tsutsui-Kimura, I., Lee, J., Guo, C., Banerjee, A., Lee, J., Amo, R., Xie, Y., Patriarchi, T., Li, Y., Watabe-Uchida, M., Uchida, N., & Kaeser, P.* (2024)
Dopamine dynamics are dispensable for movement but promote reward responses.
Nature.
[Full Text]
[PDF] |
|
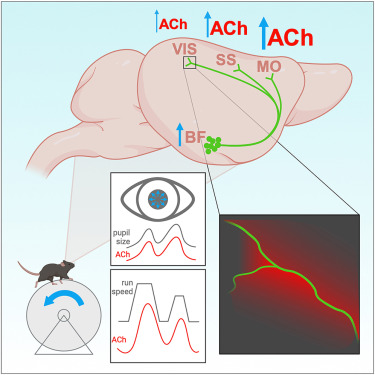 |
· Neyhart, E., Zhou, N., Munn, B., Law, R., Smith, C., Mridha, Z., Blanco, F., Li,G. , Li, Y., Hu, M., McGinley, M., Shine, J., & Reimer, J.*(2024)
Cortical acetylcholine dynamics are predicted by cholinergic axon activity and behavior state.
Cell Reports, Vol 43, Issue 10.
[Full Text]
[PDF] |
|
 |
· Berki, P., Cserép, C., Környei, Z., Pósfai, B., Szabadits, E., Domonkos, A., Kellermayer, A., Nyerges, M., Wei, X., Mody, I., Kunihiko, A., Beck, H., He K., Wang Y., Lénárt, N., Wu, Z., Jing, M., Li, Y., Gulyás, A., & Dénes, A.*(2024)
Microglia contribute to neuronal synchrony despite endogenous ATP-related phenotypic transformation in acute mouse brain slices.
Nature Communications, Vol. 15, Issue 1.
[Full Text]
[PDF] |
|
 |
· Chen, M., Ma, S., Liu, H., Dong, Y., Tang, J., Ni, Z., Tan, Y., Duan, C., Li, H., Huang, H., Li, Y., Cao, X., J. Lingle, C., Yang Y., & Hu, H.*(2024)
Brain region–specific action of ketamine as a rapid antidepressant.
Science, Vol 385, Issue 6709.
[Full Text]
[PDF] |
|
 |
· Mondoloni, S., Molina, P., Lecca, S., Wu C., Michel, L., Osypenko, D., Cachin, F., Flanigan, M., Congiu, M., L. Lalive, A., Kash, T., Deng, F., Li, Y., & Mameli, M.*(2024)
Serotonin release in the habenula during emotional contagion promotes resilience.
Science, 1081-1086 .
[Full Text]
[PDF] |
|
 |
· Singh, S., Sarroza, D., English, A., Whittington, D., Dong, A., Malamas, M., Makriyannis, A., van der Stelt, M., Li, Y., Zweifel, L., Bruchas, M. R., Land, B. B., & Stella, N.* (2024)
P2X7 receptor-dependent increase in endocannabinoid 2-arachidonoyl glycerol production by neuronal cells in culture: Dynamics and mechanism.
British Journal of Pharmacology, 1–19.
[Full Text]
[PDF] |
|
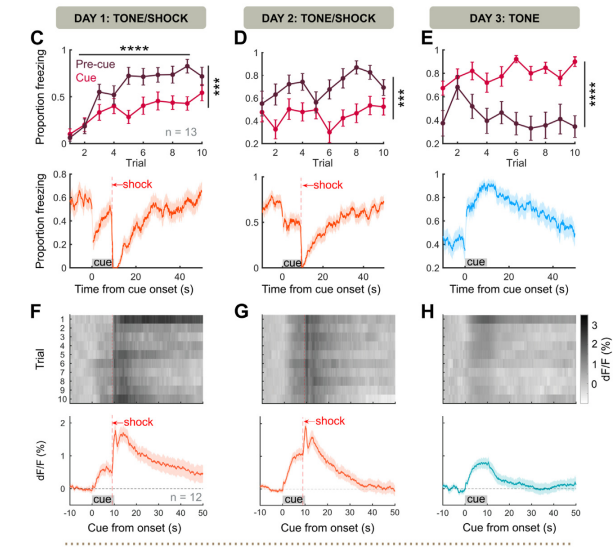 |
· Basu, A., Yang, J.-H., Yu, A., Glaeser-Khan, S., Rondeau, J. A., Feng, J., Krystal, J. H., Li, Y., & Kaye, A. P.* (2024).
Frontal Norepinephrine Represents a Threat Prediction Error Under Uncertainty.
Biological Psychiatry.
[Full Text]
[PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2022.10.13.511463 |
|
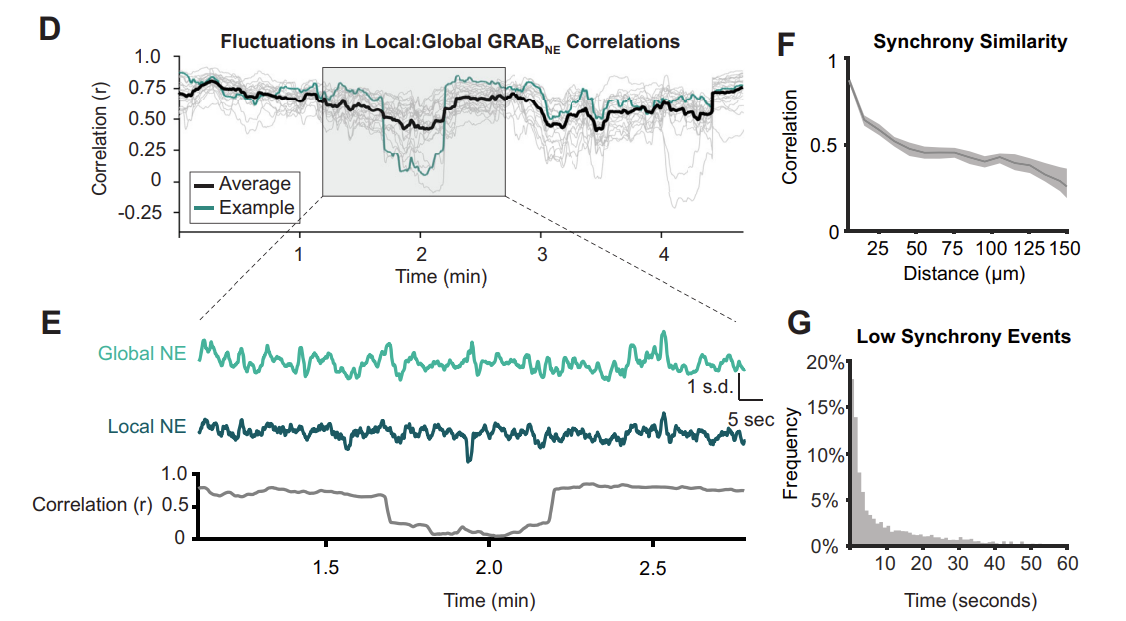 |
· Glaeser-Khan, S., Savalia, N., Cressy, J., Jiesi Feng, Li, Y., Kwan, A., & Kaye, A.* (2024).
Spatiotemporal organization of prefrontal norepinephrine influences neuronal activity.
eNeuro. 11(5).
[Full Text]
[PDF] |
|
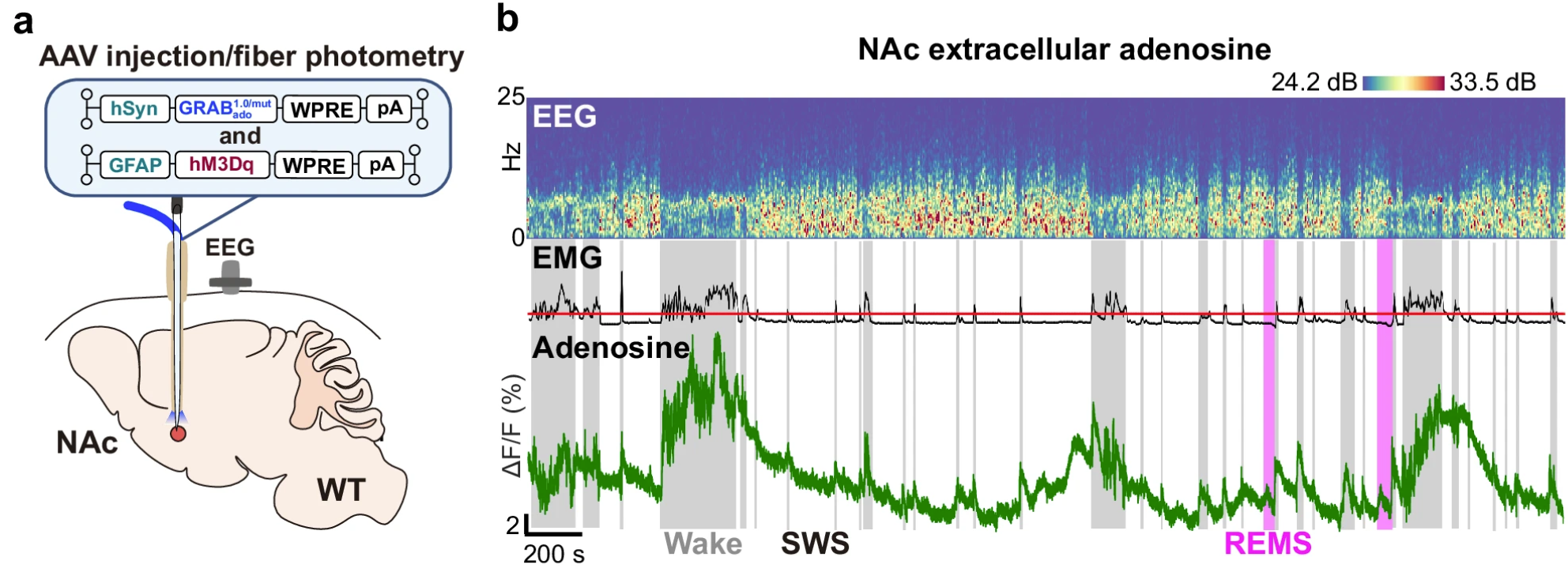 |
· Roy, K., Zhou, X., Otani, R., Yuan, P., Ioka, S., Vogt, K., Kondo, T., Farag, N., Ijiri, H., Wu, Z., Chitose, Y., Amezawa, M., Uygun, D., Cherasse, Y., Nagase, H., Li, Y., Yanagisawa, M., Abe, M., Basheer, R., Wang, Y.*, Saitoh, T.*, & Lazarus, M.* (2024).
Optochemical control of slow-wave sleep in the nucleus accumbens of male mice by a photoactivatable allosteric modulator of adenosine A2A receptors.
Nature Communications. 15, 3661.
[Full Text]
[PDF] |
|
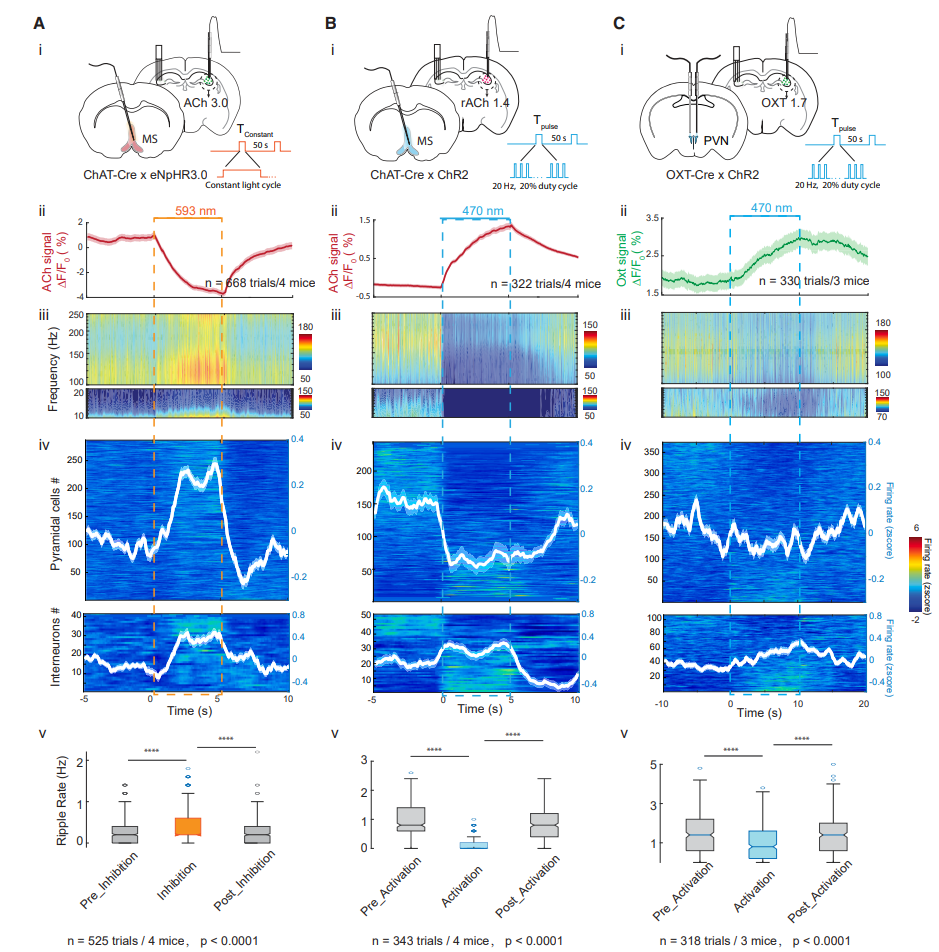 |
· Zhang, Y.#, Karadas, M.#, Liu, J., Gu, X., Vöröslakos, M., Li, Y., Tsien, R. W., & Buzsáki, G.* (2024).
Interaction of acetylcholine and oxytocin neuromodulation in the hippocampus.
Neuron, S0896-6273(24)00154-5.
[Full Text] [PDF] |
|
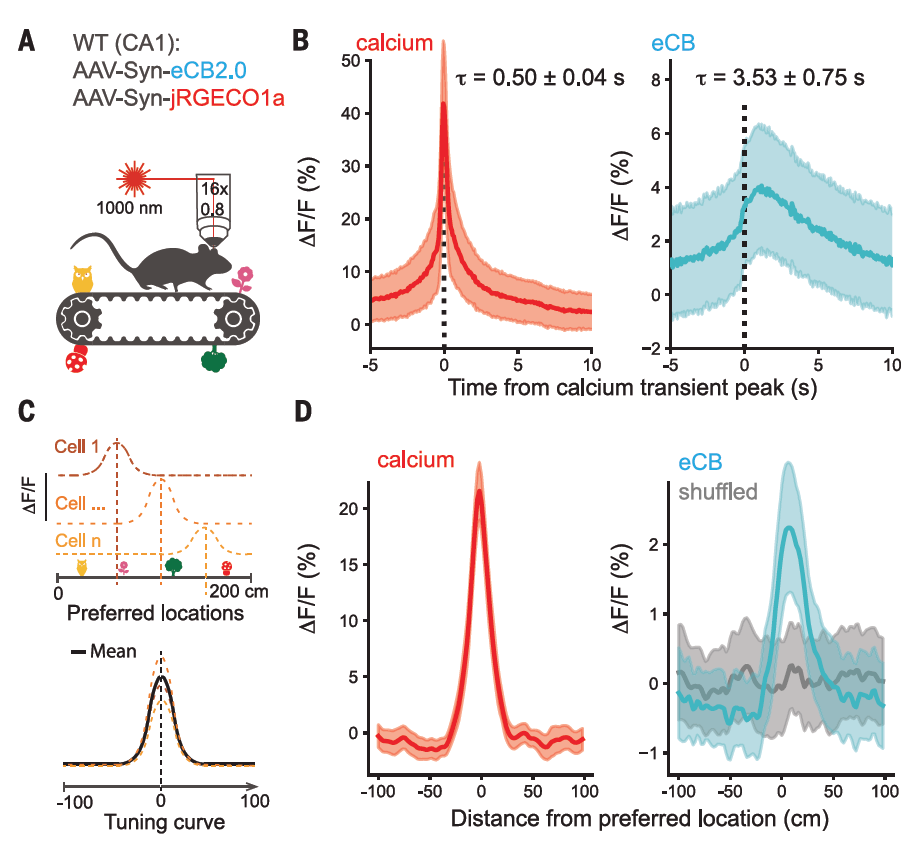 |
· Dudok, B.#*, Fan, L. Z.#, Farrell, J. S., Malhotra, S., Homidan, J., Kim, D. K., Wenardy, C., Ramakrishnan, C., Li, Y., Deisseroth, K., & Soltesz, I.* (2024).
Retrograde endocannabinoid signaling at inhibitory synapses in vivo.
Science, 383(6686), 967-970.
[Full Text] [PDF] |
|
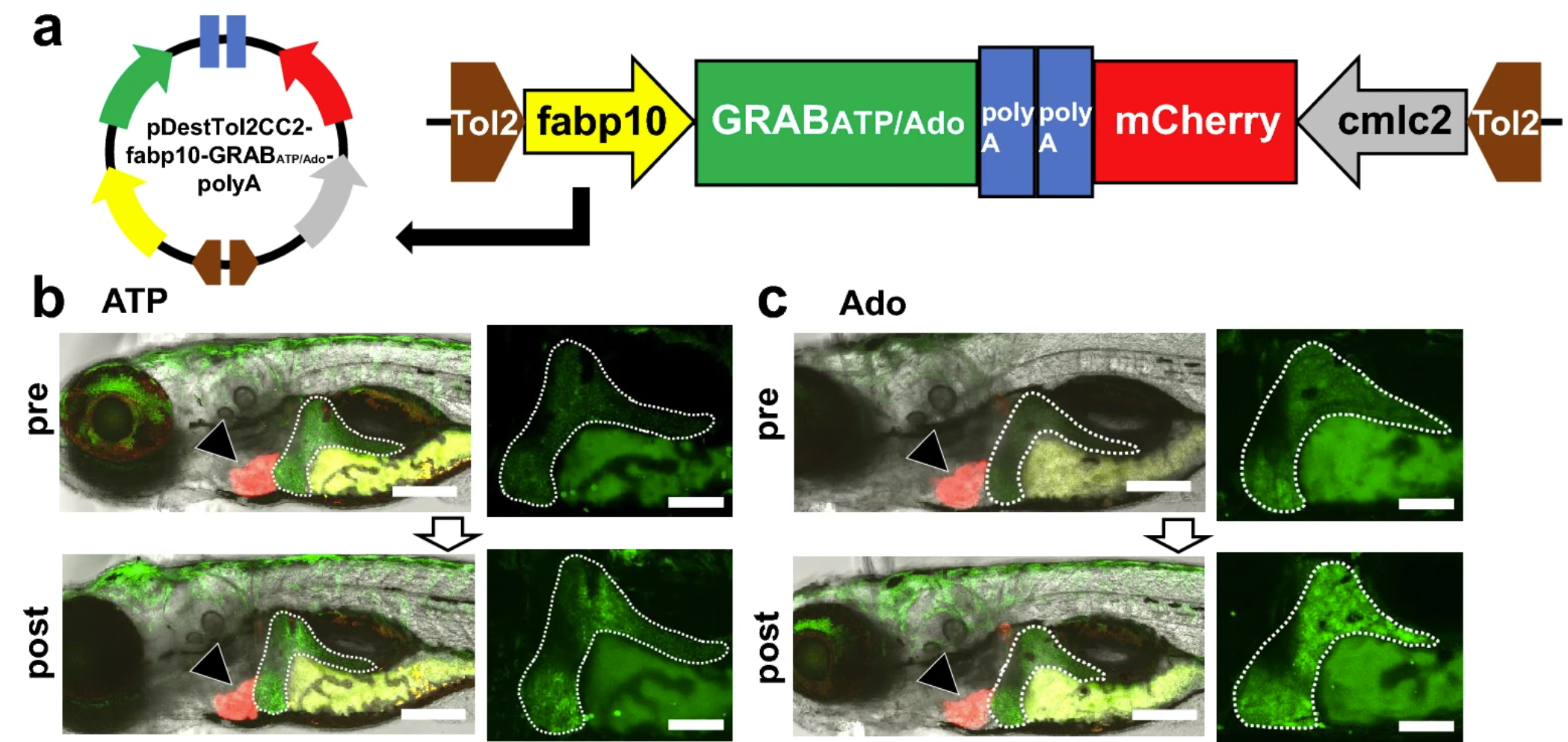 |
· Tokumaru, T., Apolinario, M., Shimizu, N., Umeda, R., Honda, K., Shikano, K., Teranishi, H., Hikida, T., Hanada, T., Ohta, K., Li, Y., Murakami, K., & Hanada, R.* (2024).
Hepatic extracellular ATP/adenosine dynamics in zebrafish models of alcoholic and metabolic steatotic liver disease.
Scientific Reports, 14, 7813.
[Full Text]
[PDF] |
|
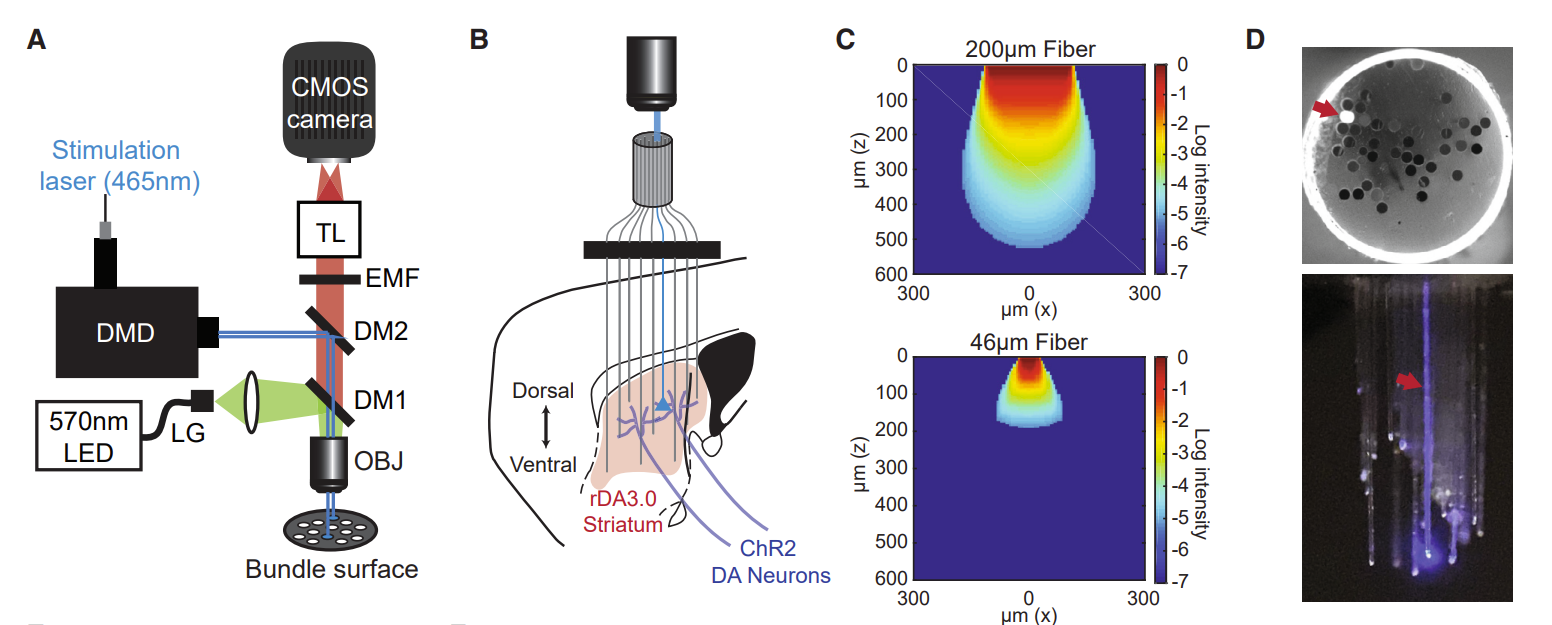 |
· Vu, M., Brown, E., Wen, M., Noggle, C., Zhang, Z,, Monk, K., Bouabid, S., Mroz, L., Graham, B., Zhuo, Y., Li, Y., Otchy, T., Tian, L, Davison, I., Boas, D., & Howe, M.* (2024).
Targeted micro-fiber arrays for measuring and manipulating localized multi-scale neural dynamics over large, deep brain volumes during behavior.
Neuron, Vol 112, Issue 6.
[Full Text]
[PDF] |
|
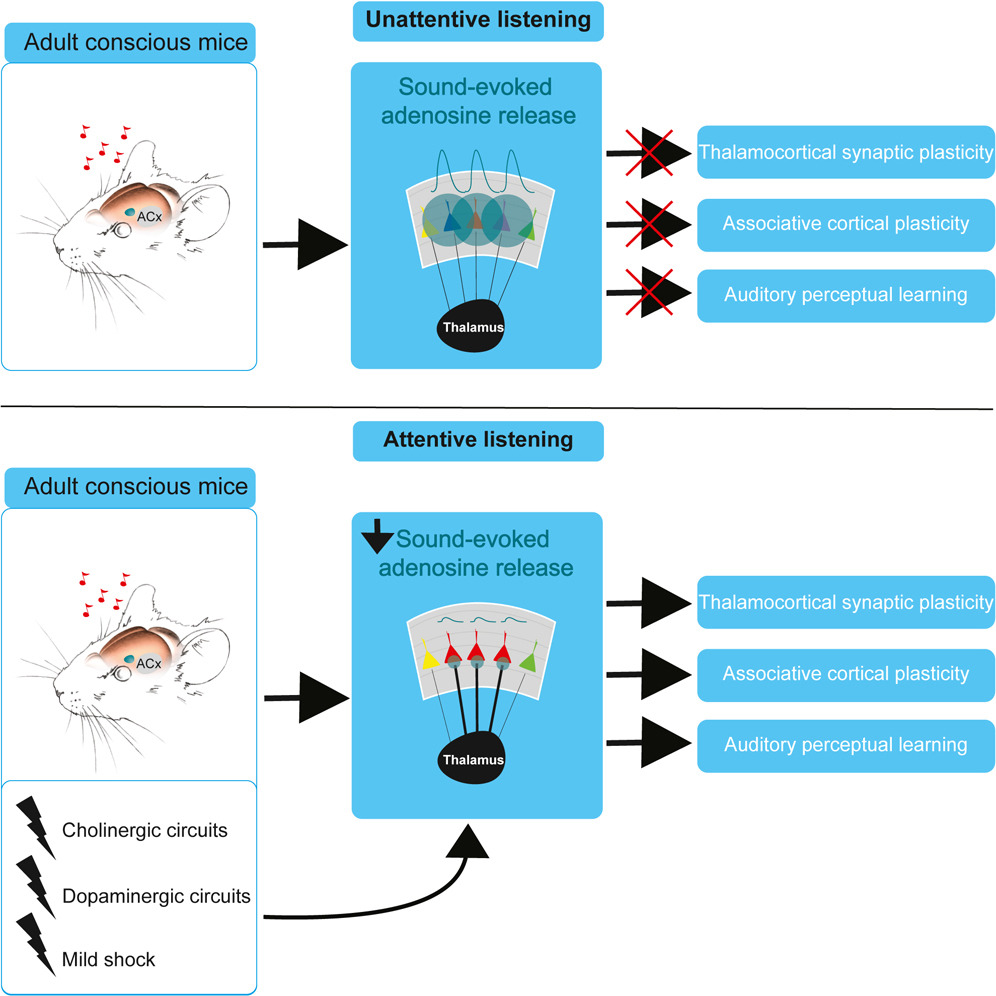 |
· Bayazitov, I., Teubner, B., Feng F., Wu, Z., Li, Y., Blundon, J., & Zakharenko, S.* (2024).
Sound-evoked adenosine release in cooperation with neuromodulatory circuits permits auditory cortical plasticity and perceptual learning.
Cell Reports, Vol 43, Issue 2.
[Full Text]
[PDF] |
|
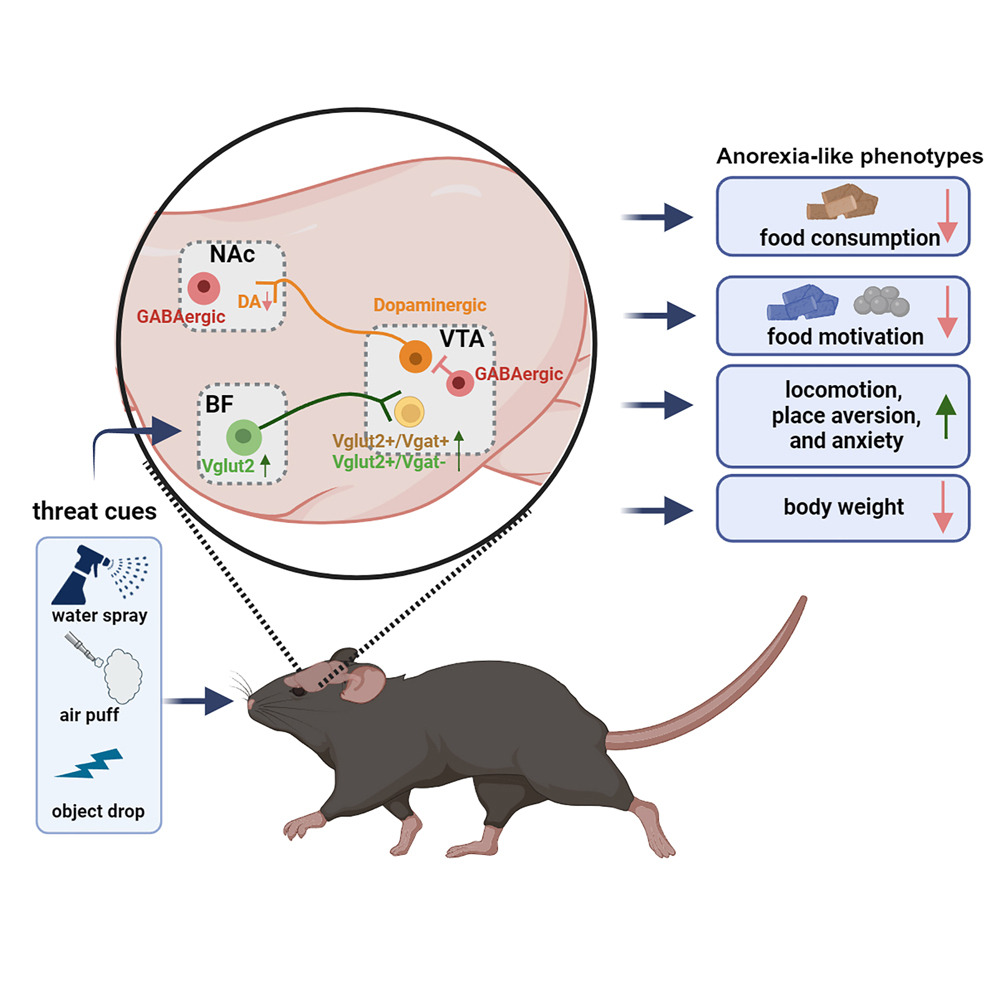 |
· Cai, J., Jiang, Y., Xu, Y., Jiang, Z., Young, C., Hongli L., Ortiz-Guzman, J., Zhuo, Y,, Li, Y., Yong X., Arenkiel, B., & Tong, Q.* (2024).
An excitatory projection from the basal forebrain to the ventral tegmental area that underlies anorexia-like phenotypes.
Neuron.. Vol 112, Issue 3.
[Full Text]
[PDF] |
|
 |
· Zhou, X.#, He, Y.#, Xu, T.#, Wu, Z.#, Guo, W., Xu, X., Liu, Y., Zhang, Y., Shang, H., Huang, L., Yao, Z., Li, Z., Su, L., Li, Z., Feng, T., Zhang, S., Monteiro, O., Cunha, R. A., Huang, Z.-L., Zhang, K.*, Li, Y., Cai, X.*, Qu, J.*, & Chen, J.-F.* (2024).
40 Hz light flickering promotes sleep through cortical adenosine signaling.
Cell Research..
[Full Text]
[PDF] |
|
 |
· Gyawali, U., Martin, D. A., Sun, F., Li, Y., & Calu, D.* (2023).
Dopamine in the dorsal bed nucleus of stria terminalis signals Pavlovian sign-tracking and reward violations.
eLife, 12.
[Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2022.06.21.497039 |
|
 |
· Mayer, F. P.*, Niello, M., Cintulova, D., Sideromenos, S., Maier, J., Li, Y., Bulling, S., Kudlacek, O., Schicker, K., Iwamoto, H., Deng, F., Wan, J., Holy, M., Katamish, R., Sandtner, W., Li, Y., Pollak, D. D., Blakely, R. D., Mihovilovic, M. D., Baumann, M. H., & Sitte, H. H.* (2023).
Serotonin-releasing agents with reduced off-target effects.
Molecular Psychiatry, 28(2), 722-732.
[Full Text] [PDF] See also BioRxiv https://www.researchsquare.com/article/rs-1886596/v1 |
|
 |
· Hatashita, Y., Wu, Z., Fujita, H., Kumamoto, T., Livet, J., Li, Y., Tanifuji, M., & Inoue, T.* (2023).
Spontaneous and multifaceted ATP release from astrocytes at the scale of hundreds of synapses.
Glia , 71(9), 2250-2265.
[Full Text] [PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2022.09.09.507300 |
|
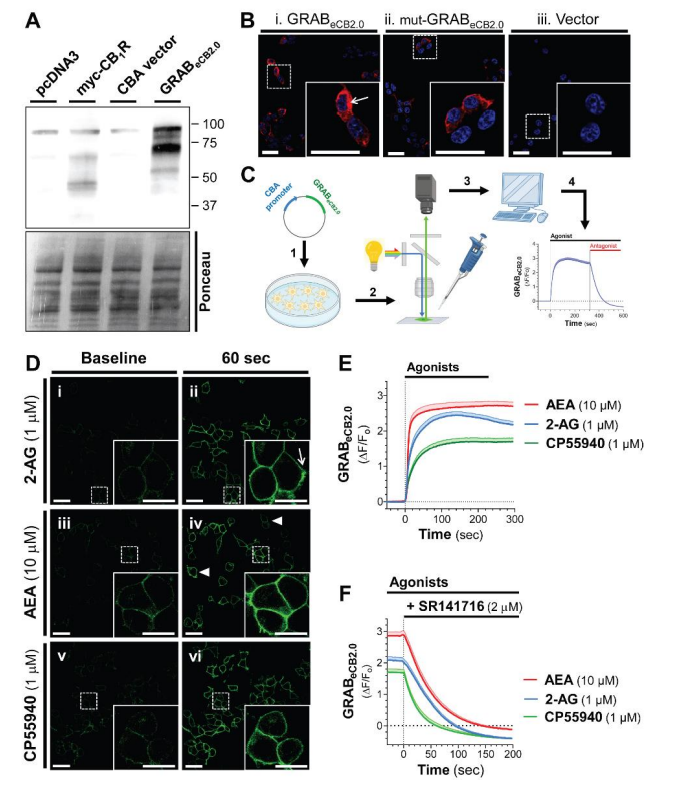 |
· Singh, S., Sarroza, D., English, A., McGrory, M., Dong, A., Zweifel, L., Land, B. B., Li, Y., Bruchas, M. R., & Stella, N.* (2023).
Pharmacological Characterization of the Endocannabinoid Sensor GRABeCB2.0.
Cannabis and Cannabinoid Research..
[Full Text] [PDF] |
|
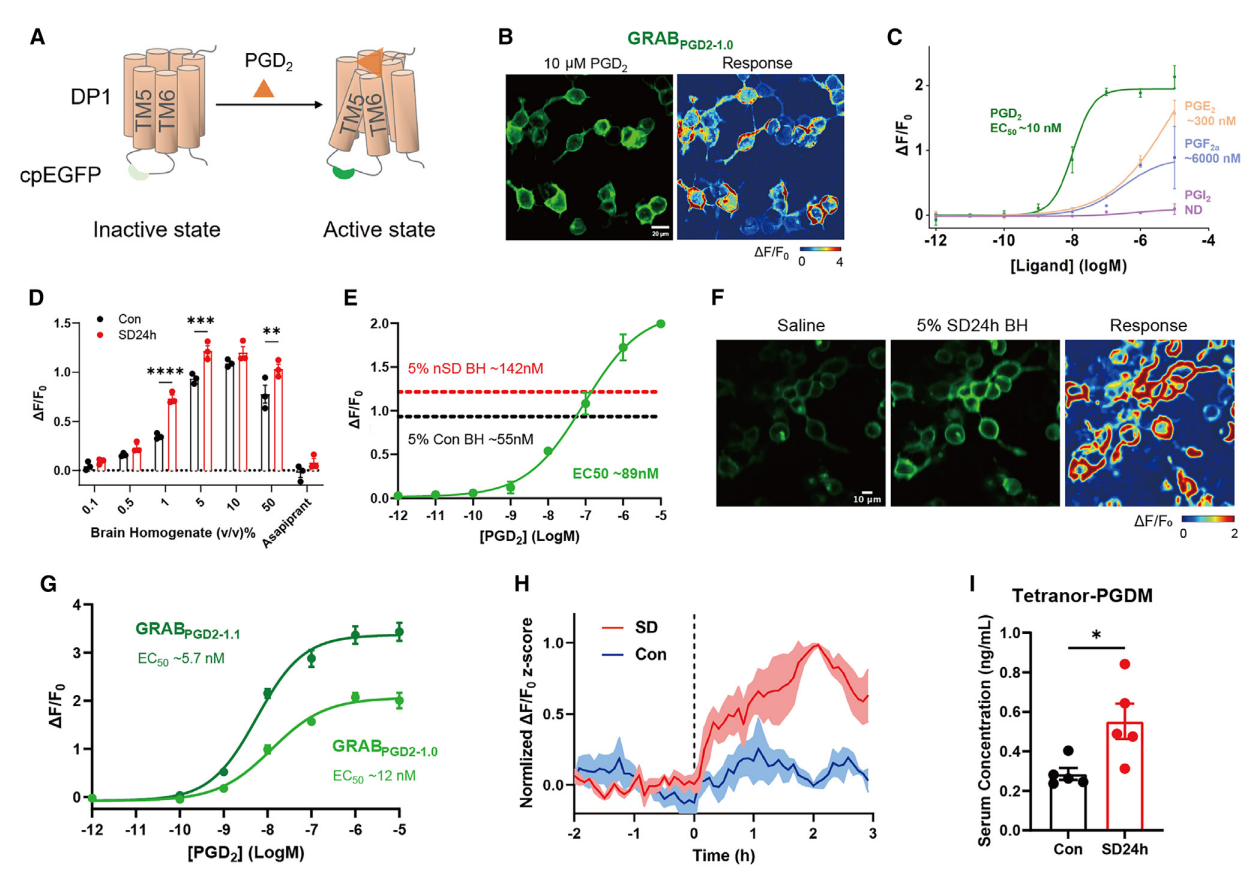 |
· Sang, D.#, Lin, K.#, Yang, Y.#, Ran, G., Li, B., Chen, C., Li, Q., Ma, Y., Lu, L., Cui, X.-Y., Liu, Z., Lv, S.-Q., Luo, M., Liu, Q., Li, Y., & Zhang, E. E.* (2023).
Prolonged sleep deprivation induces a cytokine-storm-like syndrome in mammals.
Cell, 186(25), 5500-5516.e5521.
[Full Text] [PDF] |
|
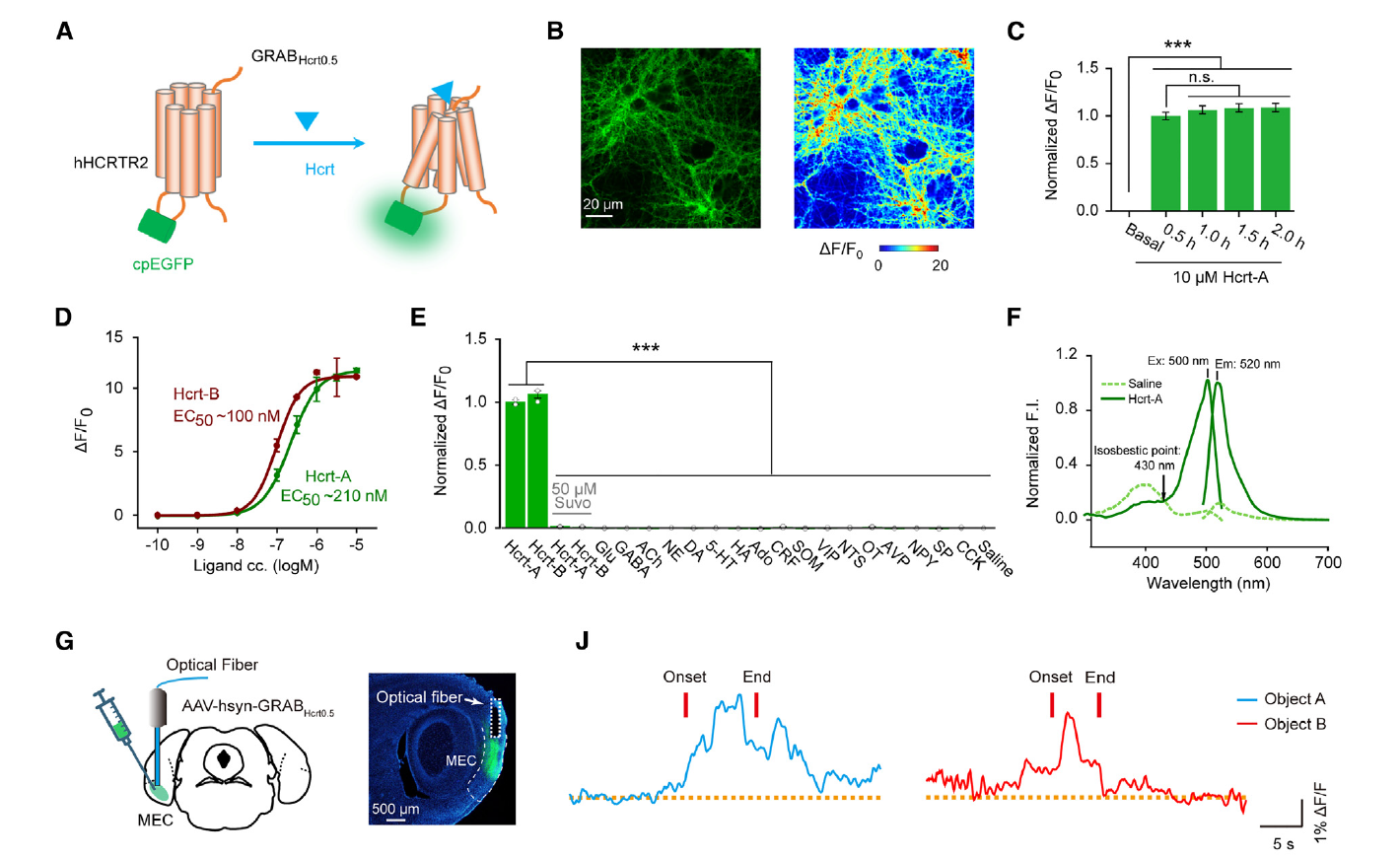 |
· Liao, Y.#, Wen, R.#, Fu, S., Cheng, X., Ren, S., Lu, M., Qian, L., Luo, F., Wang, Y., Xiao, Q., Wang, X., Ye, H., Zhang, X., Jiang, C., Li, X., Li, S., Dang, R., Liu, Y., Kang, J., Yao, Z., Yan, J., Xiong, J., Wang, Y., Wu, S., Chen, X., Li, Y., Xia, J.*, Hu, Z.*, & He, C.* (2023)
Spatial memory requires hypocretins to elevate medial entorhinal gamma oscillations.
Neuron. .
[Full Text] [PDF] |
|
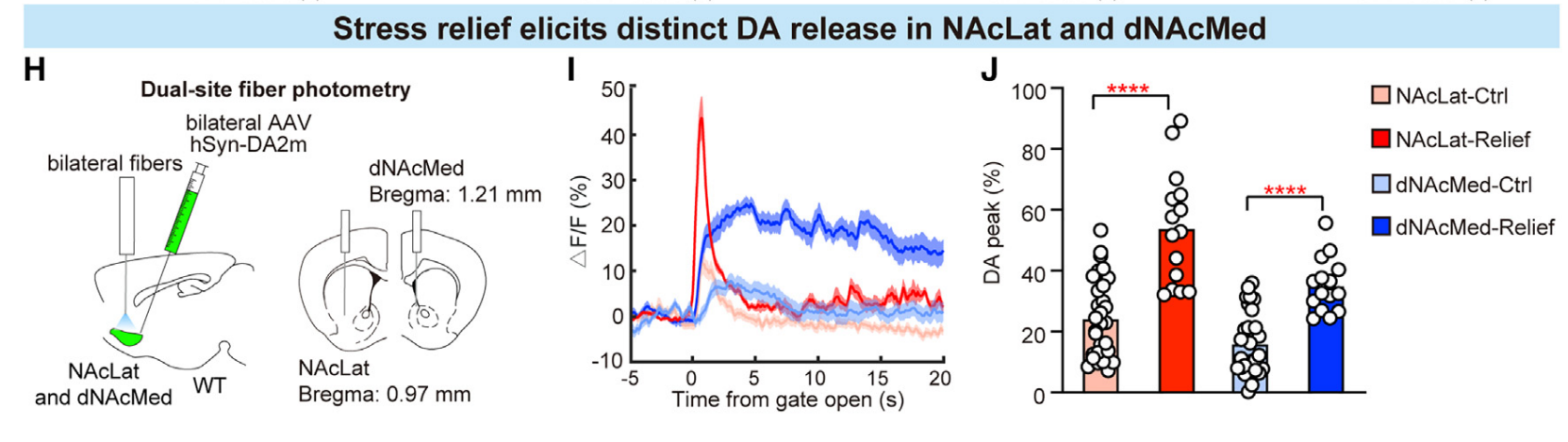 |
· Dong, Y., Li, Y., Xiang, X., Xiao, Z. C., Hu, J., Li, Y., Li, H., & Hu, H.* (2023).
Stress relief as a natural resilience mechanism against depression-like behaviors.
Neuron.
[Full Text] [PDF] |
|
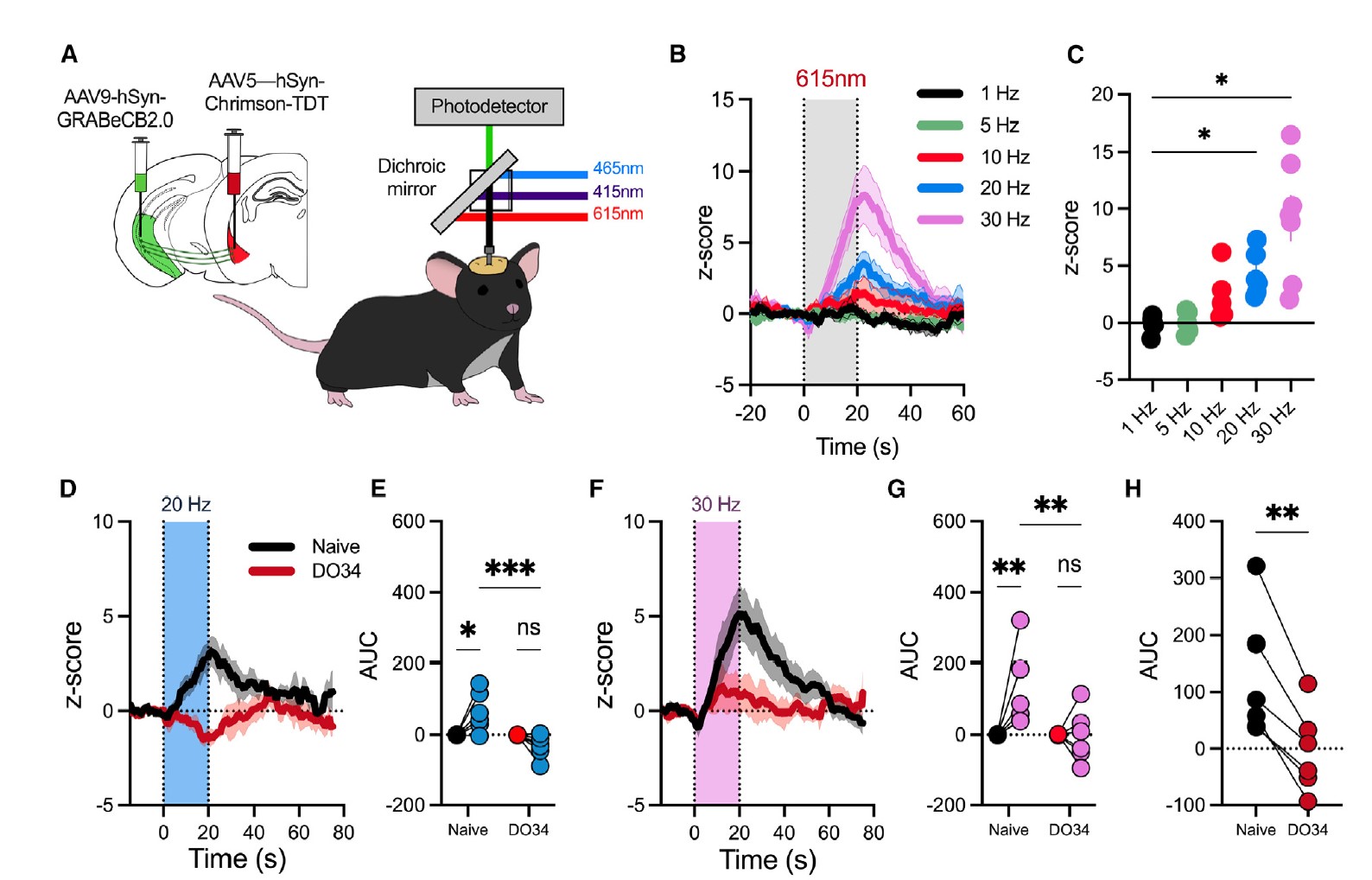 |
· Kondev, V., Najeed, M., Yasmin, F., Morgan, A., Loomba, N., Johnson, K., Adank, D. N., Dong, A., Delpire, E., Li, Y., Winder, D., Grueter, B. A., & Patel, S.* (2023).
Endocannabinoid release at ventral hippocampal-amygdala synapses regulates stress-induced behavioral adaptation.
Cell Reports, 113027.
[Full Text] [PDF] |
|
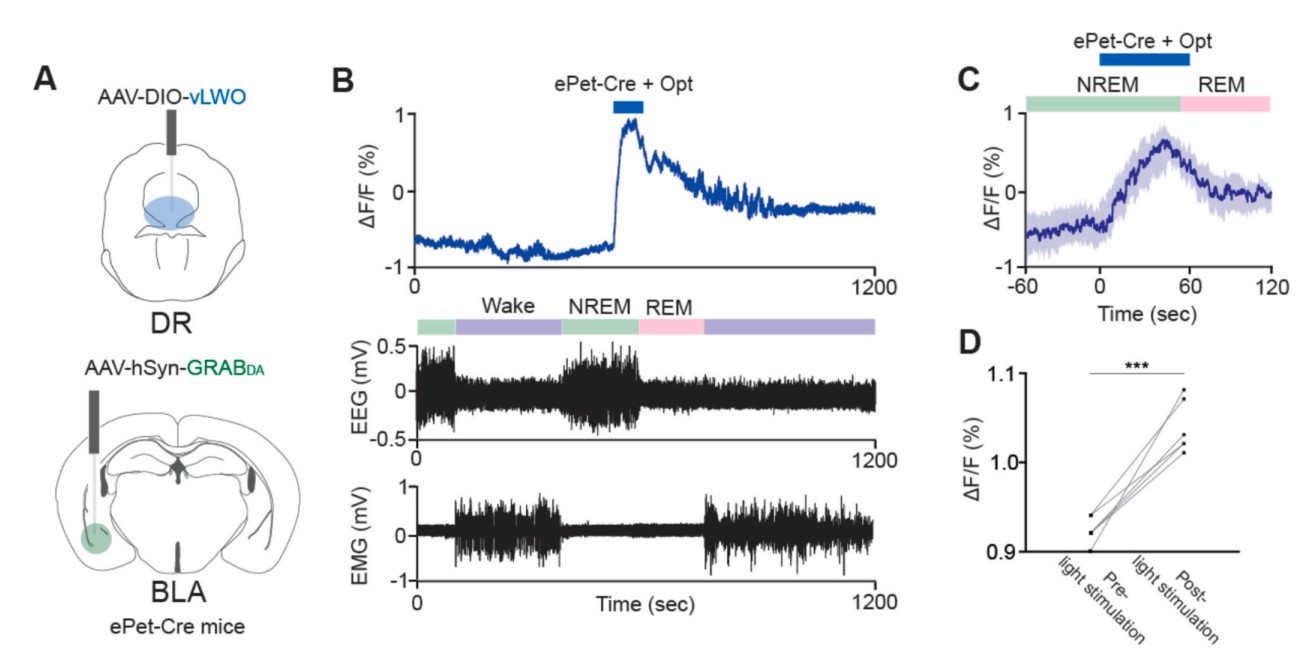 |
· Hasegawa, E., Li, Y., & Sakurai, T.* (2023).
Regulation of REM sleep in mice: The role of dopamine and serotonin function in the basolateral amygdala.
Neuroscience Research.
[Full Text] [PDF] |
|
 |
· Gunduz-Cinar, O., Castillo, L. I., Xia, M., Van Leer, E., Brockway, E. T., Pollack, G. A., Yasmin, F., Bukalo, O., Limoges, A., Oreizi-Esfahani, S., Kondev, V., Báldi, R., Dong, A., Harvey-White, J., Cinar, R., Kunos, G., Li, Y., Zweifel, L. S., Patel, S., & Holmes, A. (2023).
A cortico-amygdala neural substrate for endocannabinoid modulation of fear extinction.
Neuron..
[Full Text] [PDF] |
|
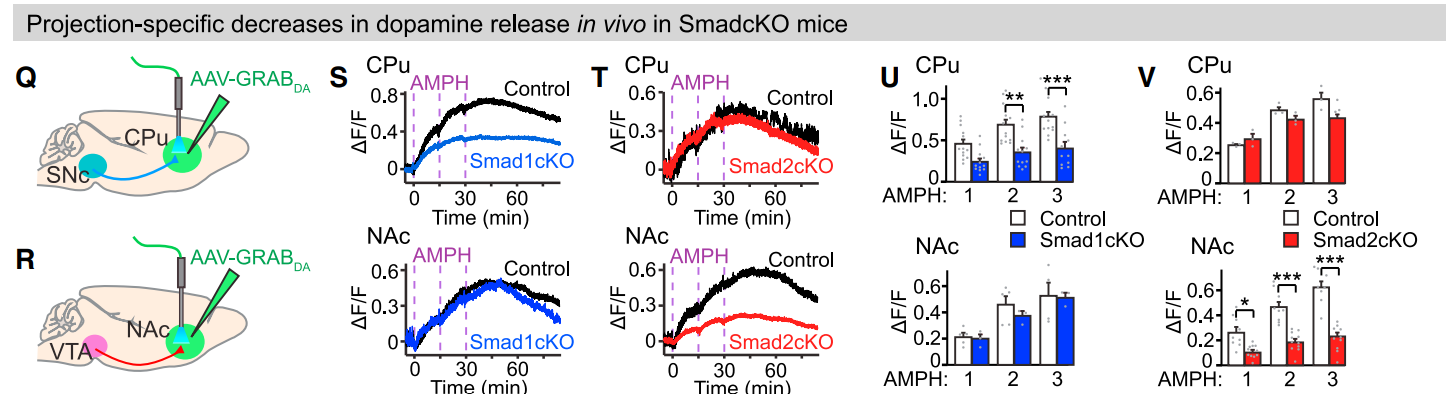 |
· Terauchi, A., Yee, P., Johnson-Venkatesh, E. M., Seiglie, M. P., Kim, L., Pitino, J. C., Kritzer, E., Zhang, Q., Zhou, J., Li, Y., Ginty, D. D., Lee, W. A., & Umemori, H.* (2023).
The projection-specific signals that establish functionally segregated dopaminergic synapses.
Cell.
[Full Text] [PDF] |
|
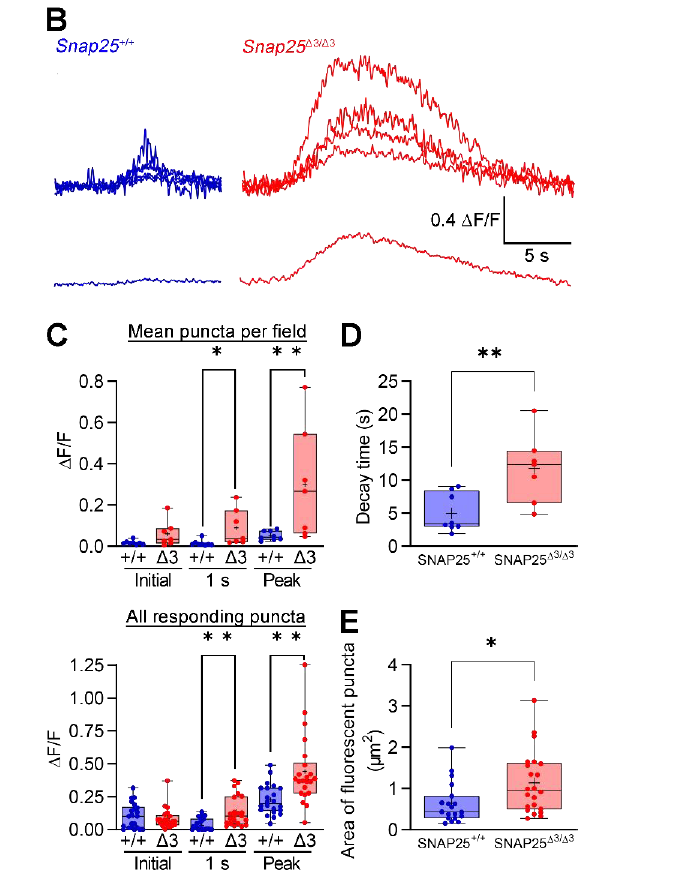 |
· Ceddia, R. P.#, Zurawski, Z.#, Thompson Gray, A., Adegboye, F., McDonald-Boyer, A., Shi, F., Liu, D., Maldonado, J., Feng, J., Li, Y., Alford, S., Ayala, J. E., McGuinness, O. P., Collins, S., & Hamm, H. E.* (2023).
Gβγ-SNAP25 exocytotic brake removal enhances insulin action, promotes adipocyte browning, and protects against diet-induced obesity.
The Journal of Clinical Investigation.
[Full Text] [PDF] |
|
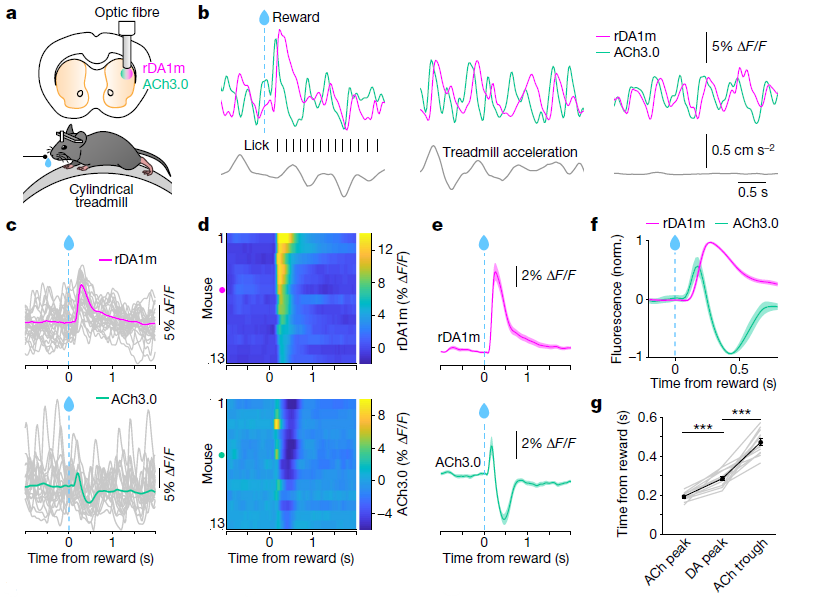 |
· Krok, A. C., Maltese, M., Mistry, P., Miao, X., Li, Y., & Tritsch, N. X.* (2023).
Intrinsic dopamine and acetylcholine dynamics in the striatum of mice.
Nature.
[Full Text] [PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2022.09.09.507300 |
|
 |
· Kimchi, E.*, Burgos-Robles, A., Matthews, G., Chakoma, T., Patarino, M., Weddington, J., Siciliano, C., Yang, W., Foutch, S., Simons, R., Fong, M., Jing, M., Li, Y., Polley, D., Tye, K.* (2023).
Reward contingency gates selective cholinergic suppression of amygdala neurons.
eLife, 12:RP89093
[Full Text] [PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2022.02.04.479188v2 |
|
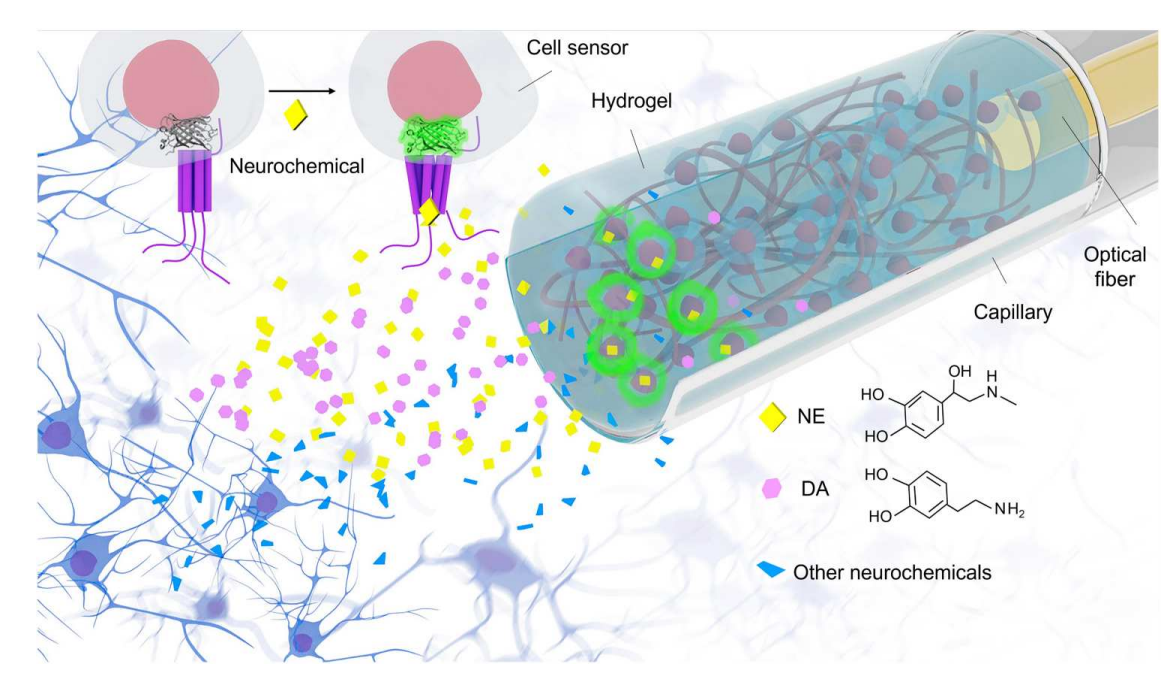 |
· Zhou, B.#, Fan, K.#, Guo, J.#, Feng, J., Yang, C., Li, Y., Shi, S., & Kong, L. (2023).
Plug-and-play fiber-optic sensors based on engineered cells for neurochemical monitoring at high specificity in freely moving animals.
Science Advances, 9(22), eadg0218.
[Full Text] [PDF] |
|
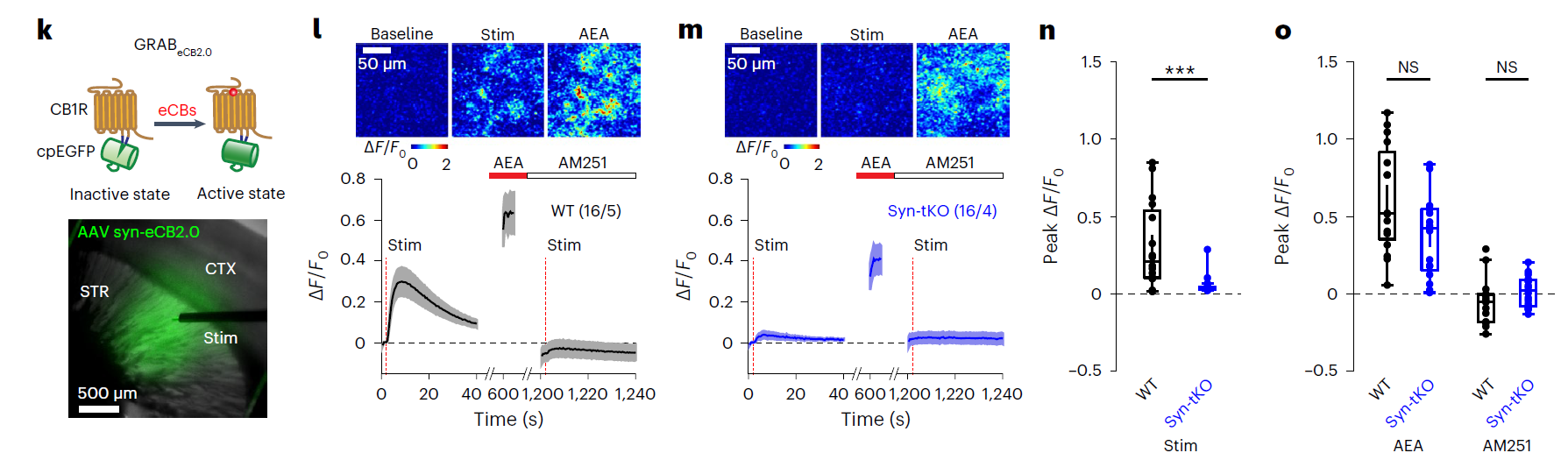 |
· Albarran, E., Sun, Y., Liu, Y., Raju, K., Dong, A., Li, Y., Wang, S., Südhof, T. C.*, & Ding, J. B.* (2023).
Postsynaptic synucleins mediate endocannabinoid signaling.
Nature Neuroscience, 26(6), 997-1007.
[Full Text] [PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2021.10.04.462870v1 |
|
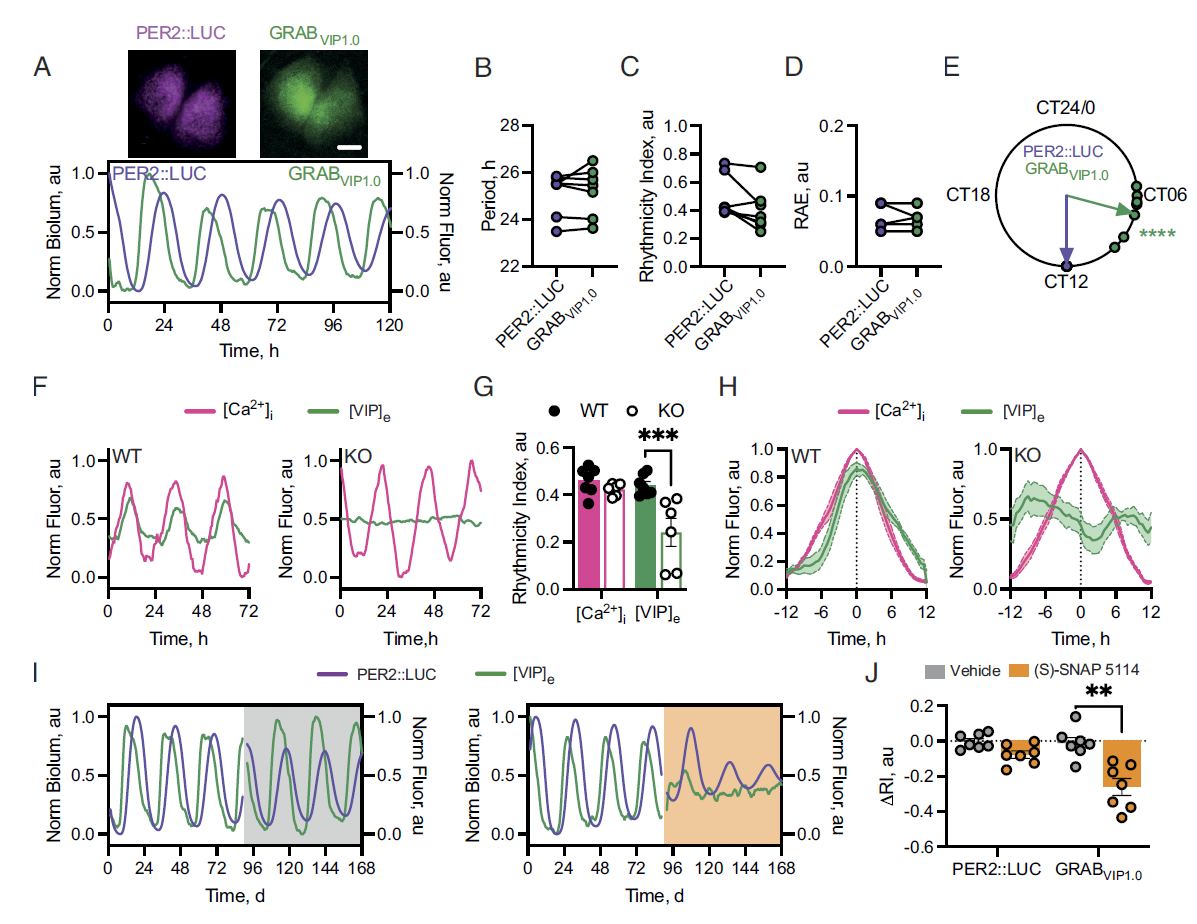 |
· Patton, A. P.*, Morris, E. L., McManus, D., Wang, H., Li, Y., Chin, J. W., & Hastings, M. H.* (2023).
Astrocytic control of extracellular GABA drives circadian timekeeping in the suprachiasmatic nucleus.
Proceedings of the National Academy of Sciences , 120(21), e2301330120.
[Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2023.01.16.523253 |
|
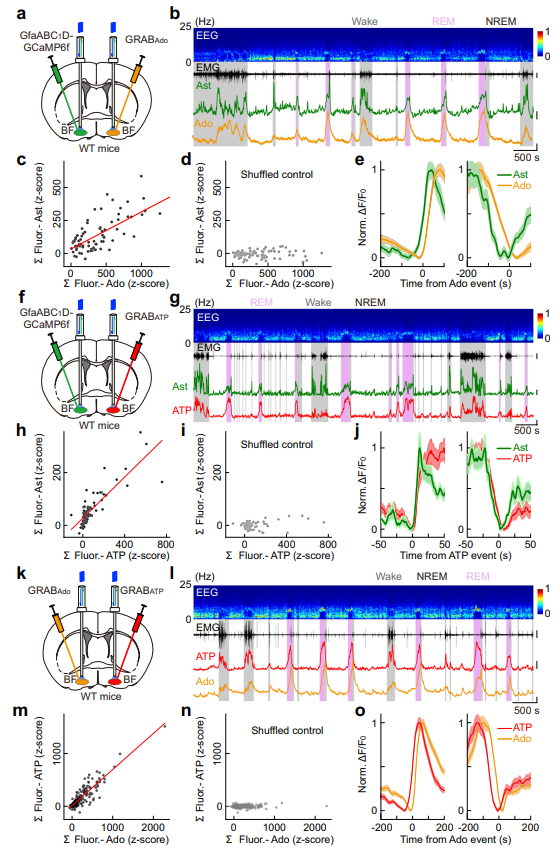 |
· Peng, W.*, Liu, X., Ma, G., Wu, Z., Wang, Z., Fei, X., Qin, M., Wang, L., Li, Y., Zhang, S.*, & Xu, M.* (2023).
Adenosine-independent regulation of the sleep–wake cycle by astrocyte activity.
Cell Discovery, 9(1), 16.
[Full Text] [PDF] |
|
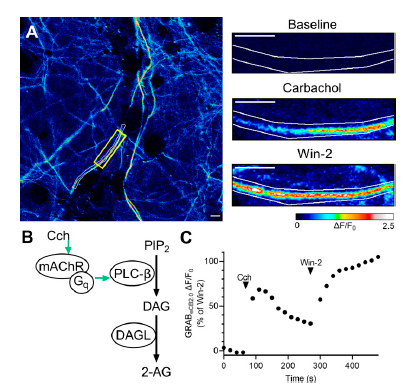 |
· Asher, M. J., McMullan, H. M., Dong, A., Li, Y., & Thayer, S. A.* (2023)
A Complete Endocannabinoid Signaling System Modulates Synaptic Transmission between Human Induced Pluripotent Stem Cell-Derived Neurons.
Mol Pharmacol , 103(2), 100-112.
[Full Text] [PDF] |
|
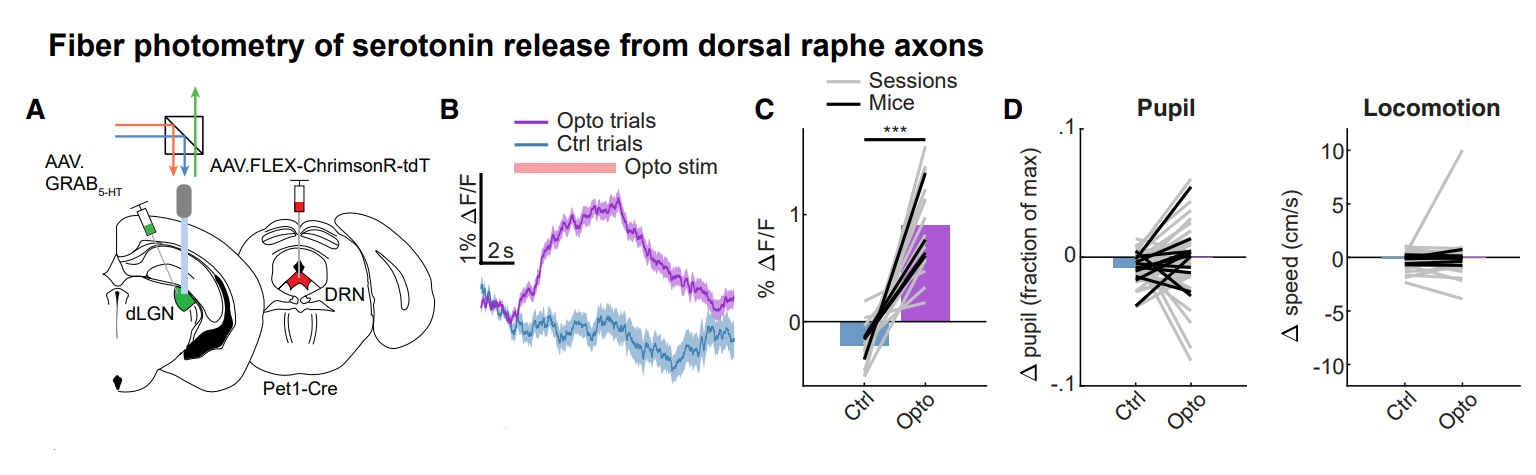
 |
· Natsubori, A.*, Hirai, S., Kwon, S., Ono, D., Deng, F., Wan, J., Miyazawa, M., Kojima, T., Okado, H., Karashima, A., Li, Y., Tanaka, K. F., & Honda, M. (2023).
Serotonergic neurons control cortical neuronal intracellular energy dynamics by modulating astrocyte-neuron lactate shuttle.
iScience, 105830.
[Full Text] [PDF] |
|
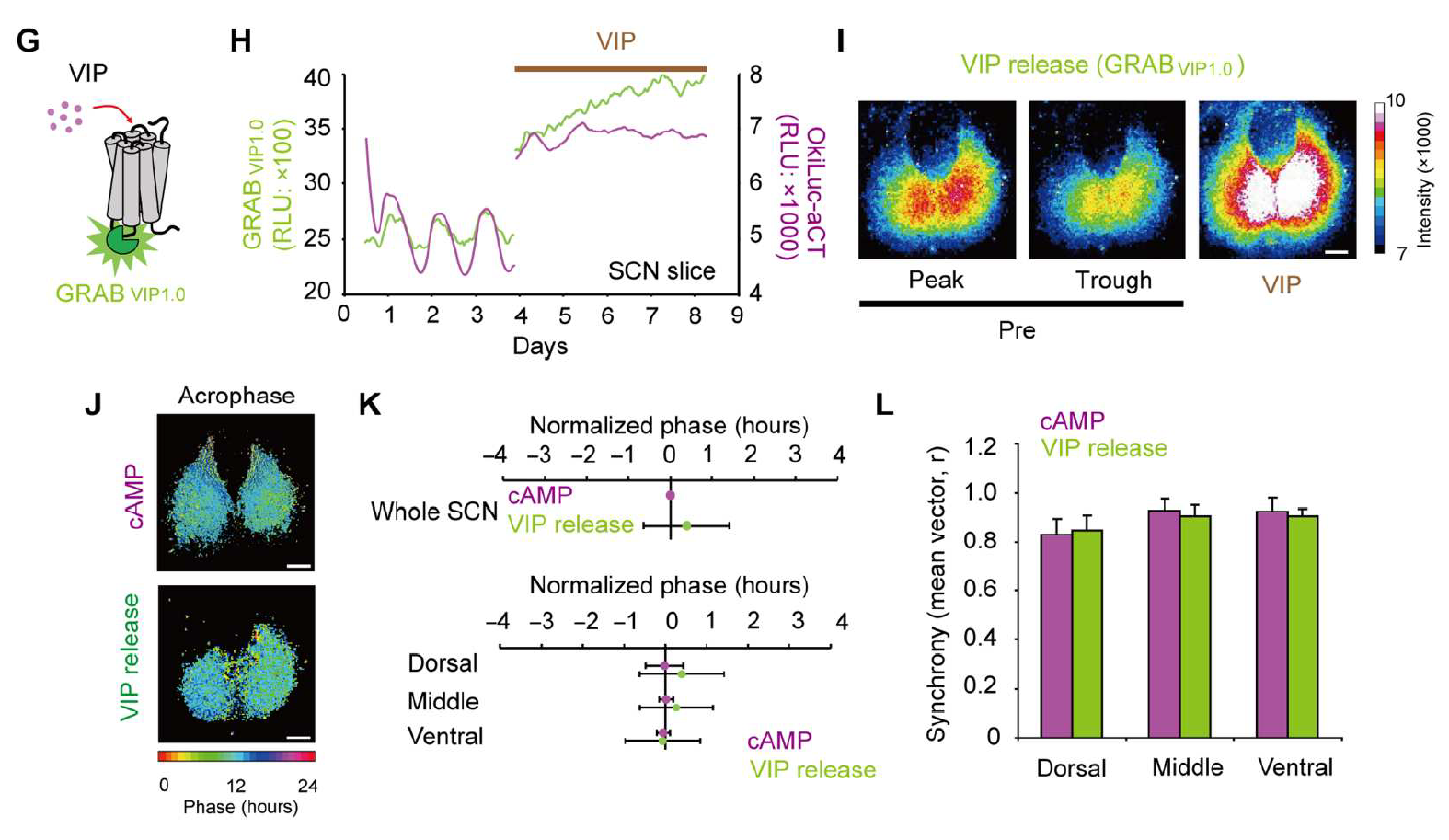 |
· Ono, D.*, Wang, H., Hung, C. J., Wang, H.-t., Kon, N., Yamanaka, A.,Li, Y., & Sugiyama, T.
Network-driven intracellular cAMP coordinates circadian rhythm in the suprachiasmatic nucleus.
Science Advances, 9(1), eabq7032.
[Full Text] [PDF] |
|
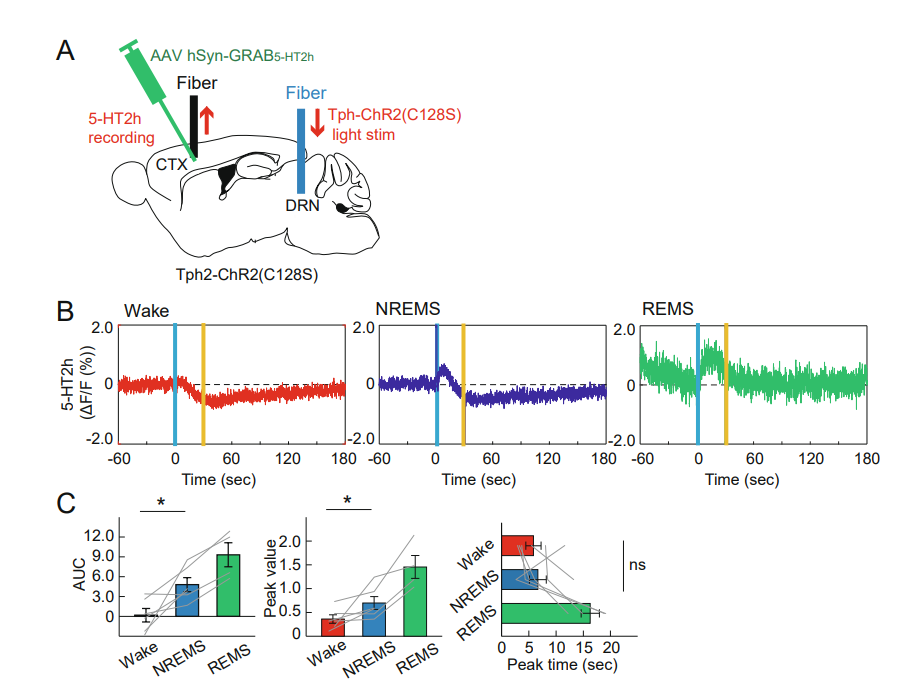
 |
· Reggiani, J. D. S., Jiang, Q., Barbini, M., Lutas, A., Liang, L., Fernando, J., Deng, F., Wan, J., Li, Y., Chen, C.*, & Andermann, M. L.* (2022).
Brainstem serotonin neurons selectively gate retinal information flow to thalamus.
Neuron.
[Full Text] [PDF] |
|
 |
· Pittolo, S., Yokoyama, S., Willoughby, D. D., Taylor, C. R., Reitman, M. E., Tse, V., Wu, Z., Etchenique, R., Li, Y., & Poskanzer, K. E.* (2022).
Dopamine activates astrocytes in prefrontal cortex via α1-adrenergic receptors.
Cell Reports, 40(13), 111426.
[Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2022.07.19.500710 |
|
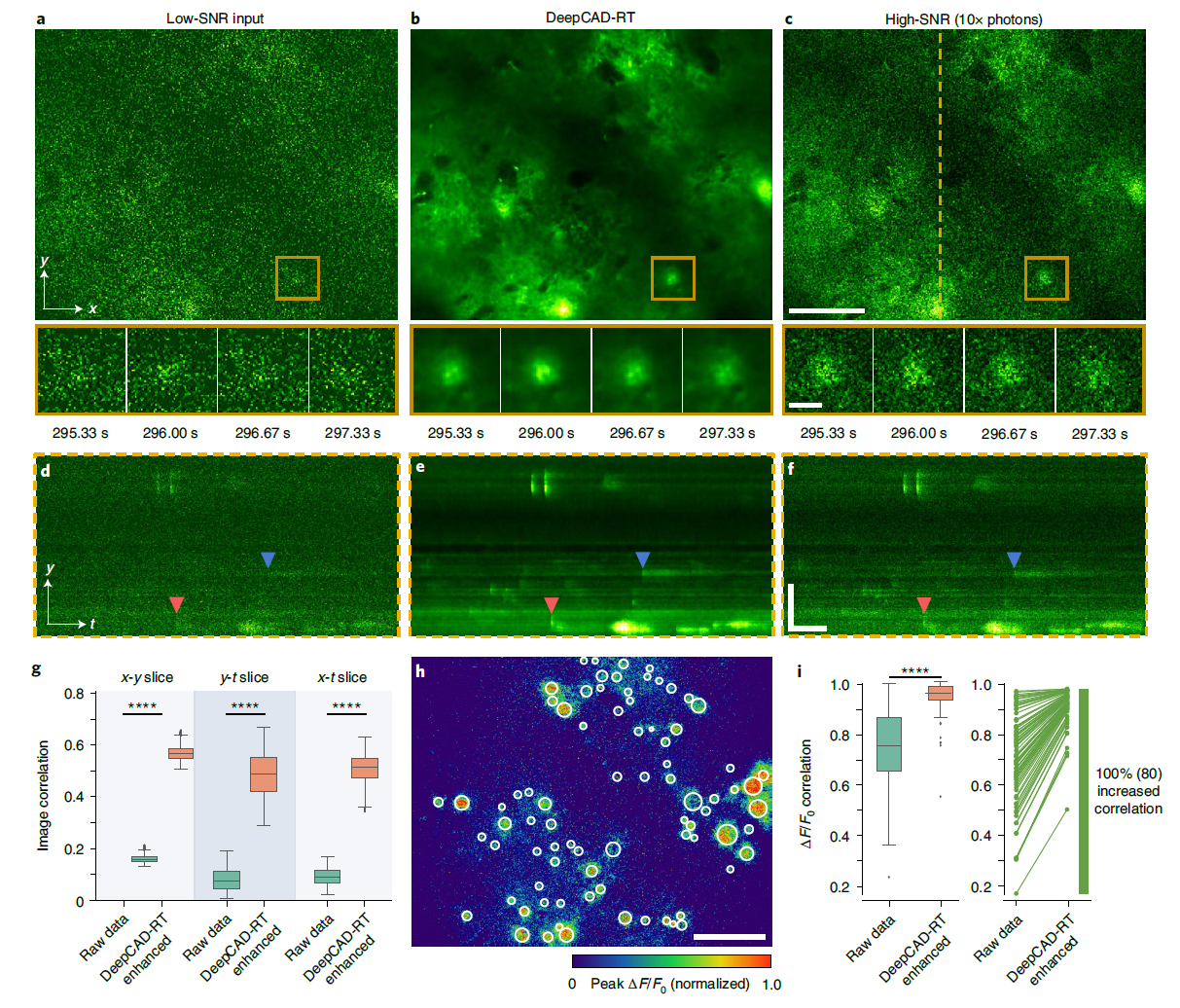 |
· Li, X.#, Li, Y.#, Zhou, Y., Wu, J., Zhao, Z., Fan, J., Deng, F., Wu, Z., Xiao, G., He, J., Zhang, Y., Zhang, G., Hu, X., Chen, X., Zhang, Y., Qiao, H., Xie, H., Li, Y., Wang, H.*, Fang, L.*, & Dai, Q.* (2022).
Real-time denoising enables high-sensitivity fluorescence time-lapse imaging beyond the shot-noise limit.
Nature Biotechnology.
[Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2022.03.14.484230 |
|
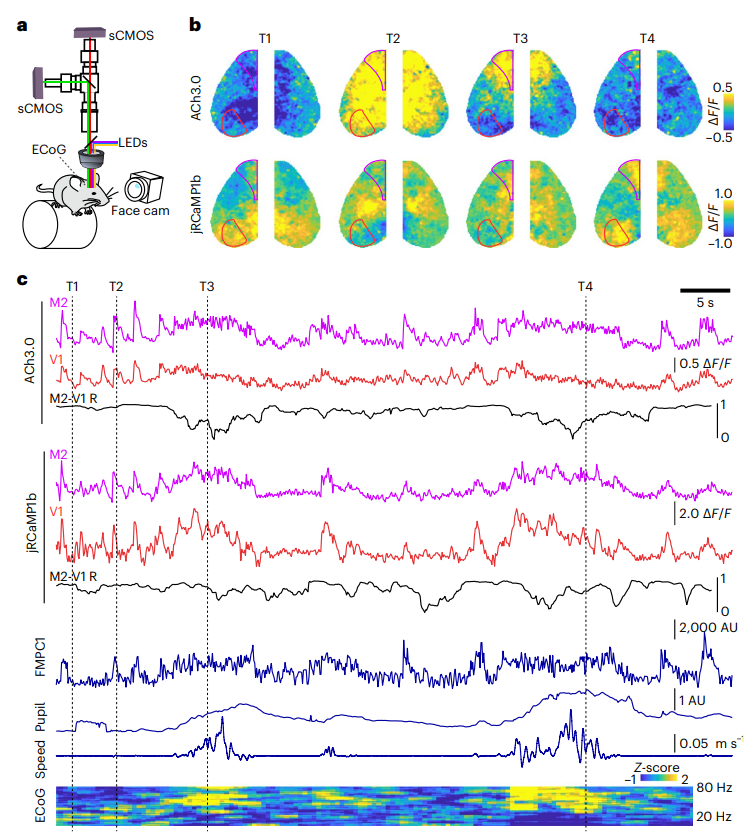 |
· Lohani, S.#, Moberly, A. H.#, Benisty, H., Landa, B., Jing, M., Li, Y., Higley, M. J.*, & Cardin, J. A.* (2022).
Spatiotemporally heterogeneous coordination of cholinergic and neocortical activity. Nature Neuroscience, 25(12), 1706-1713.
[Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2020.12.09.418632 |
|
 |
· Wang, L.#, Wu, C.#, Peng, W., Zhou, Z., Zeng, J., Li, X., Yang, Y., Yu, S., Zou, Y., Huang, M., Liu, C., Chen, Y., Li, Y., Ti, P., Liu, W., Gao, Y., Zheng, W., Zhong, H., Gao, S., Lu, Z., Ren, P.-G., Ng, H. L., He, J., Chen, S., Xu, M., Li, Y., & Chu, J.* (2022).
A high-performance genetically encoded fluorescent indicator for in vivo cAMP imaging.
Nature Communications, 13(1), 5363.
[Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2022.02.27.482140 |
|
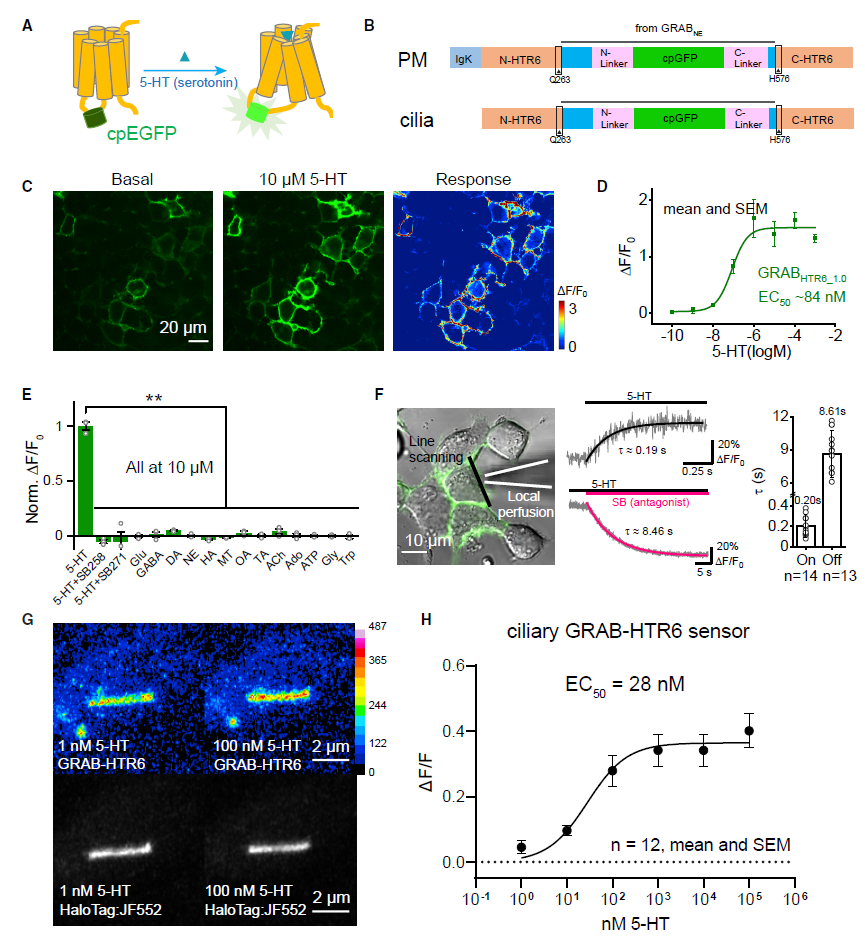 |
· Sheu, S.-H.*, Upadhyayula, S., Dupuy, V., Pang, S., Deng, F., Wan, J., Walpita, D., Pasolli, H. A., Houser, J., Sanchez-Martinez, S., Brauchi, S. E., Banala, S., Freeman, M., Xu, C. S., Kirchhausen, T., Hess, H. F., Lavis, L., Li, Y., Chaumont-Dubel, S., & Clapham, D. E.* (2022).
A serotonergic axon-cilium synapse drives nuclear signaling to alter chromatin accessibility. Cell, 185(18), 3390-3407.e3318.
[Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2021.09.27.461878 * See Preview by: Simon, D. J., & Levitz, J. (2022).
A ciliary synapse for “short-circuit” neuromodulation.
Cell , 185(18), 3284-3286.
[Full Text] [PDF] |
|
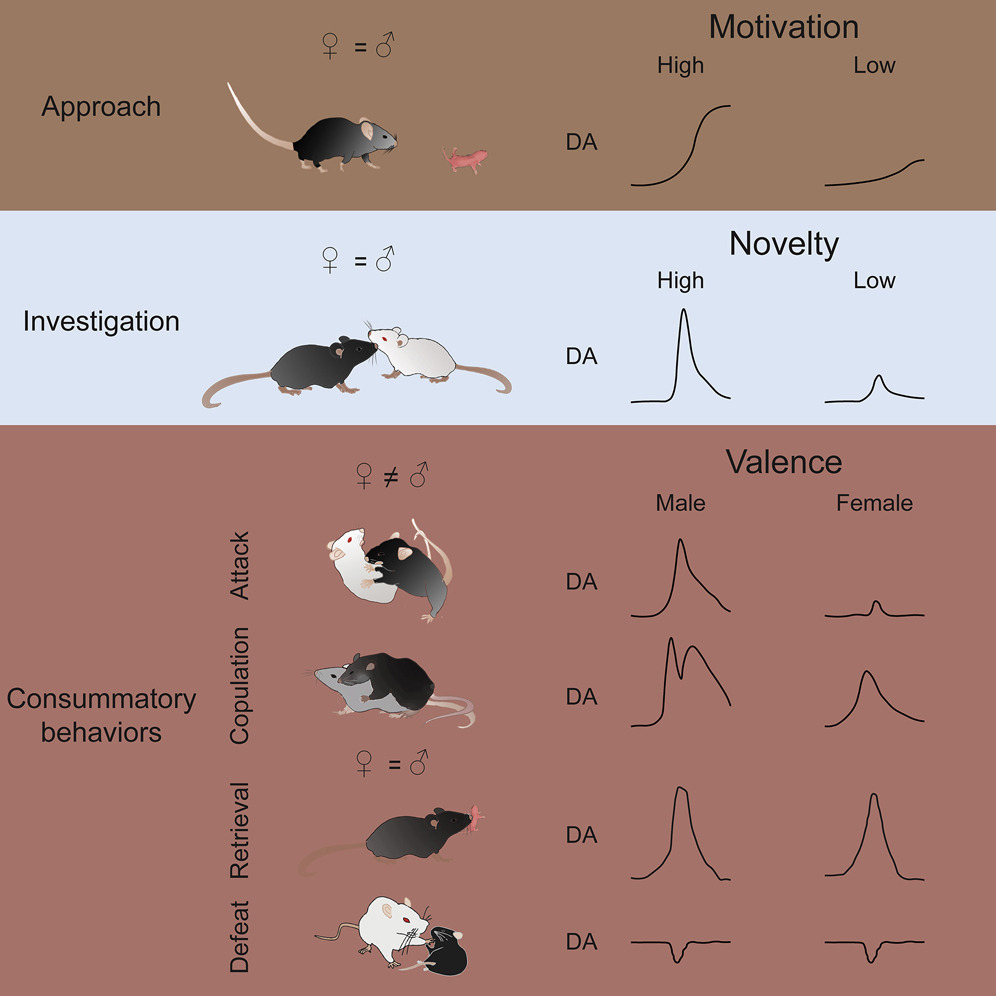 |
· Dai, B.*, Sun, F., Tong, X., Ding, Y., Kuang, A., Osakada, T., Li, Y., & Lin, D.* (2022).
Responses and functions of dopamine in nucleus accumbens core during social behaviors. Cell Reports, 40(8), 111246.
[Full Text] [PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2021.06.22.449478v1 |
|
 |
· Lin, R.*#, Zhou, Y.#, Yan, T.#, Wang, R.#, Li, H., Wu, Z., Zhang, X., Zhou, X., Zhao, F., Zhang, L., Li, Y., & Luo, M.* (2022).
Directed evolution of adeno-associated virus for efficient gene delivery to microglia. Nat Methods, 19(8), 976-985.
[Full Text] [PDF] |
|
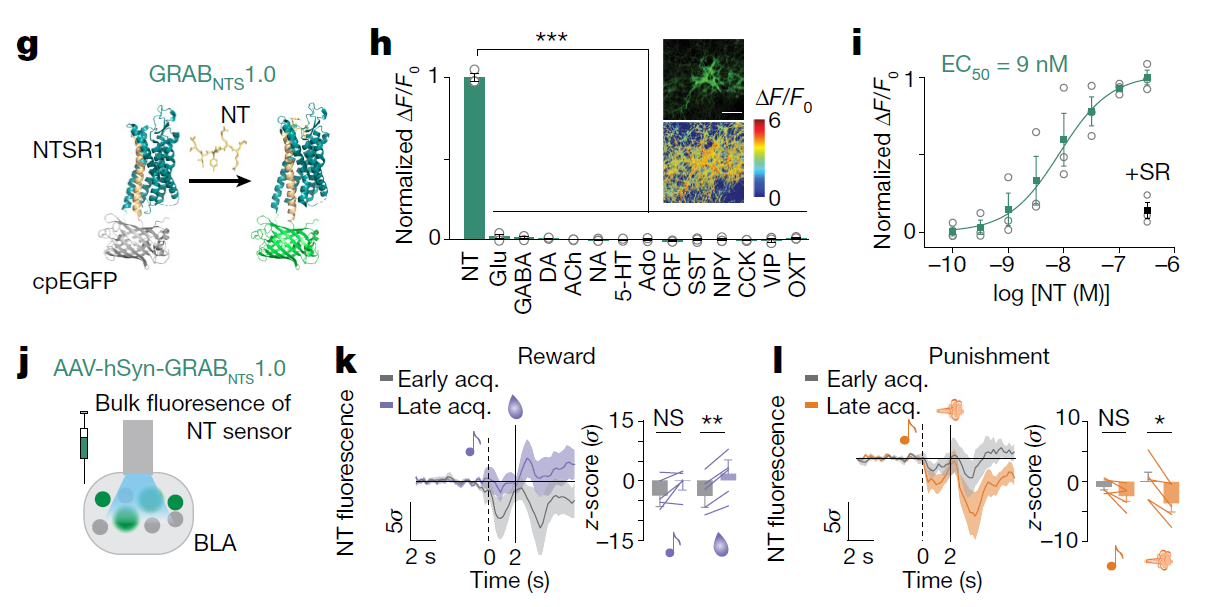 |
· Li, H.#, Namburi, P.#, Olson, J. M.#, Borio, M., Lemieux, M. E., Beyeler, A., Calhoon, G. G., Hitora-Imamura, N., Coley, A. A., Libster, A., Bal, A., Jin, X., Wang, H., Jia, C., Choudhury, S. R., Shi, X., Felix-Ortiz, A. C., de la Fuente, V., Barth, V. P., King, H. O., Izadmehr, E. M., Revanna, J. S., Batra, K., Fischer, K. B., Keyes, L. R., Padilla-Coreano, N., Siciliano, C. A., McCullough, K. M., Wichmann, R., Ressler, K. J., Fiete, I. R., Zhang, F., Li, Y., & Tye, K. M.* (2022).
Neurotensin orchestrates valence assignment in the amygdala. Nature.
[Full Text] [PDF] |
|
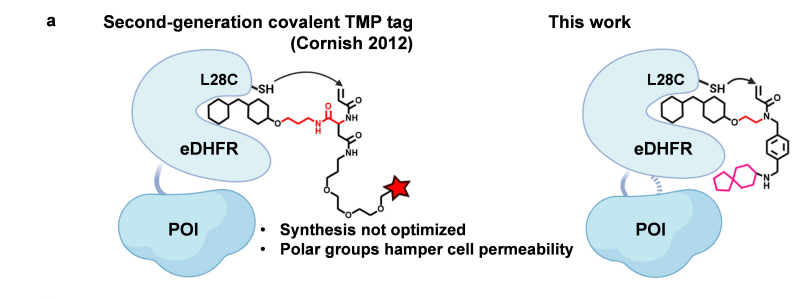 |
· Mo, J.#, Chen, J.#, Shi, Y., Sun, J., Wu, Y., Liu, T., Zhang, J., Zheng, Y., Li, Y., & Chen, Z.* (2022).
Third-Generation Covalent TMP-Tag for Fast Labeling and Multiplexed Imaging of Cellular Proteins Angewandte Chemie International Edition, e202207905.
[Full Text] [PDF] |
|
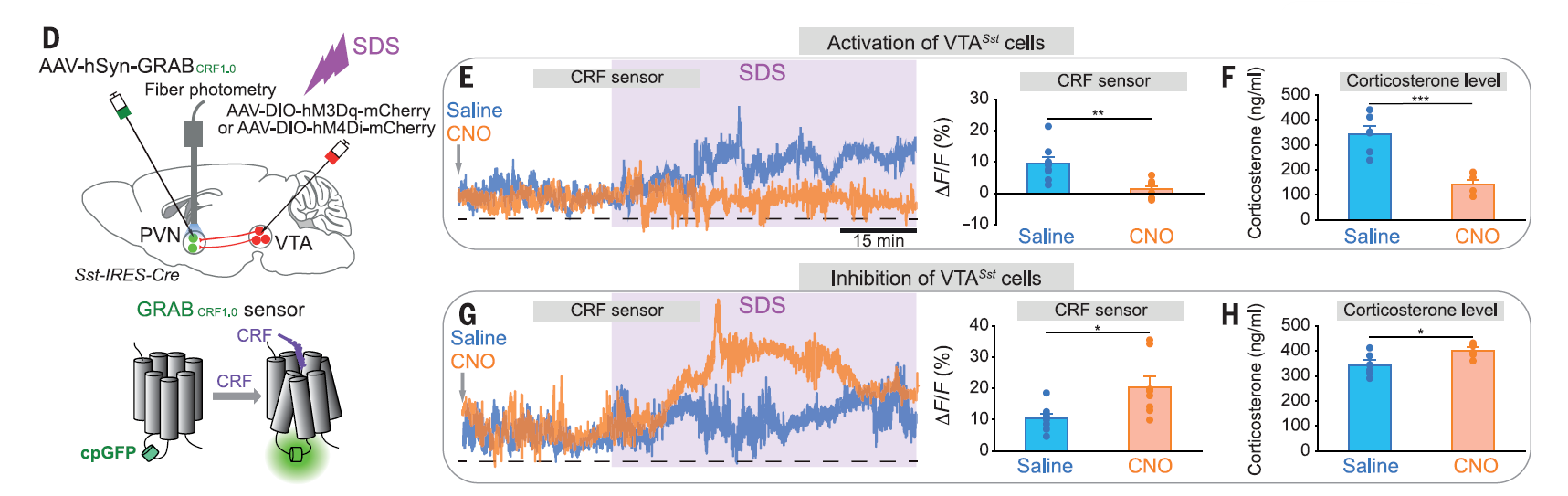 |
· Yu, X.#*, Zhao, G.#, Wang, D., Wang, S., Li, R., Li, A., Wang, H., Nollet, M., Chun, Y. Y., Zhao, T., Yustos, R., Li, H., Zhao, J., Li, J., Cai, M., Vyssotski, A. L., ,Li, Y., Dong, H.*, Franks, N. P.*, & Wisden, W.* (2022).
A specific circuit in the midbrain detects stress and induces restorative sleep. Science, 377(6601), 63-72. [Full Text] [PDF] |
|
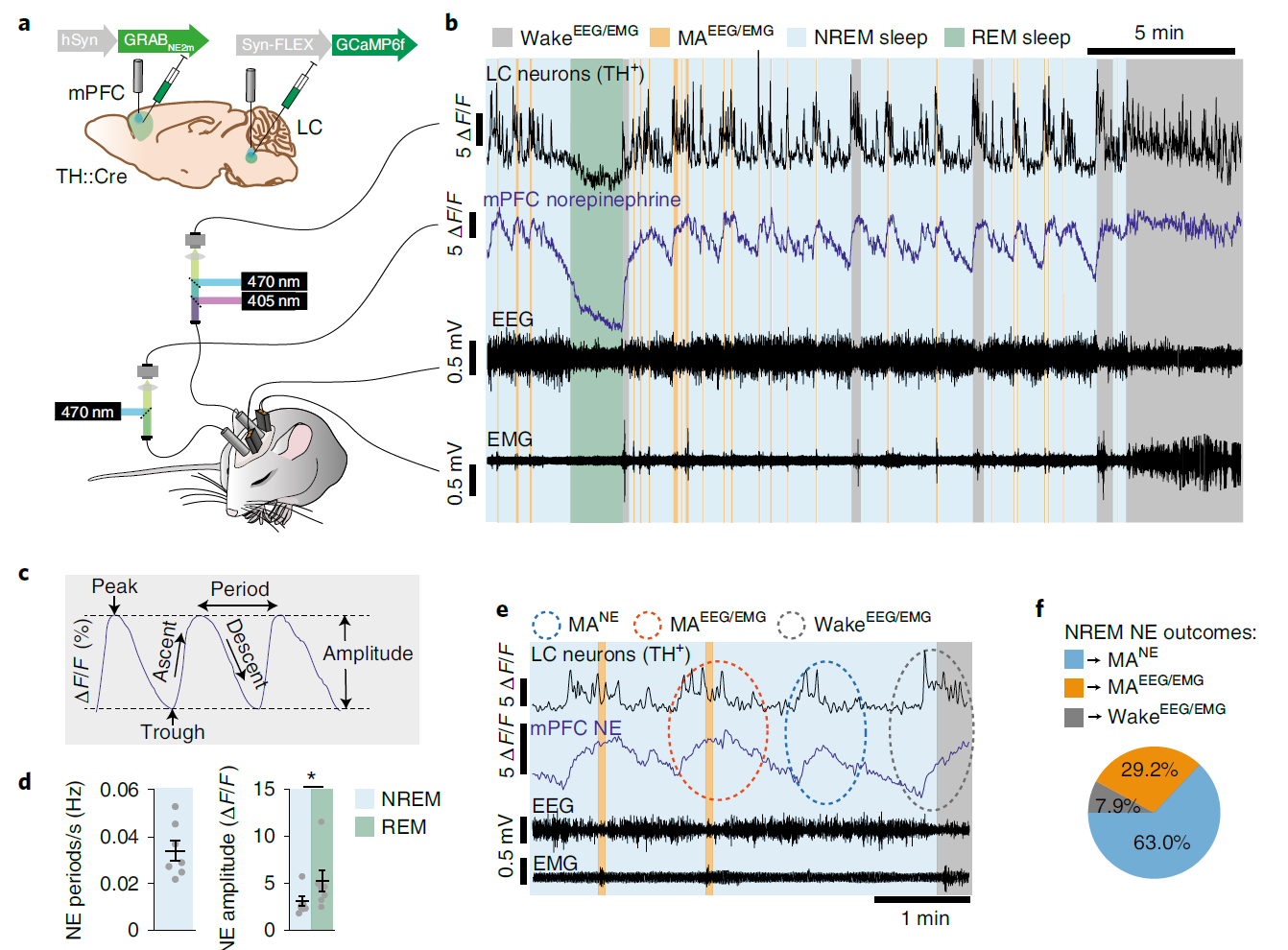 |
· Kjaerby, C.*, Andersen, M., Hauglund, N., Untiet, V., Dall, C., Sigurdsson, B., Ding, F., Feng, J., Li, Y., Weikop, P., Hirase, H., & Nedergaard, M.* (2022).
Memory-enhancing properties of sleep depend on the oscillatory amplitude of norepinephrine. Nature Neuroscience.
[Full Text] [PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2020.09.01.274977v1 * See Comments Highlight by: Morici, J. F., & Girardeau, G.* (2022).
Cortical norepinephrine GRABs a seat at the sleep table.
Nature Neuroscience, 25(8), 978-980.
[Full Text] [PDF] |
|
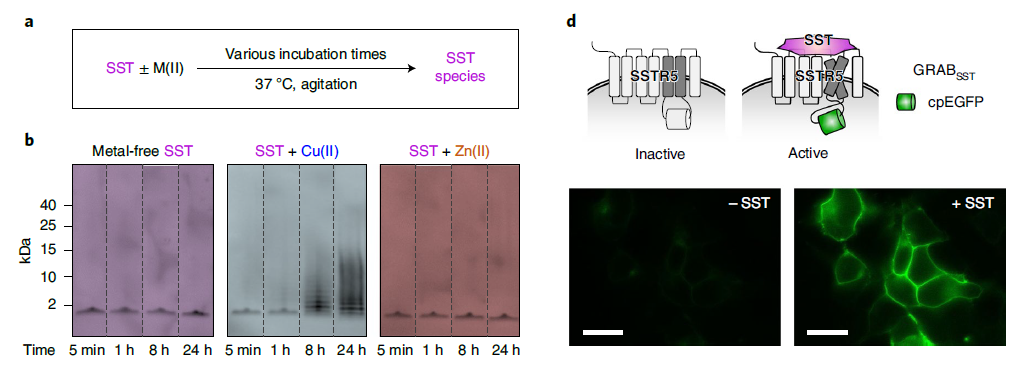 |
· Han, J., Yoon, J., Shin, J., Nam, E., Qian, T.,Li, Y., Park, K.*, Lee, S.-H.*, & Lim, M. H.* (2022).
Conformational and functional changes of the native neuropeptide somatostatin occur in the presence of copper and amyloid-β. Nature Chemistry. [Full Text] [PDF] See also ChemRxiv https://doi.org/10.26434/chemrxiv.14736882 |
|
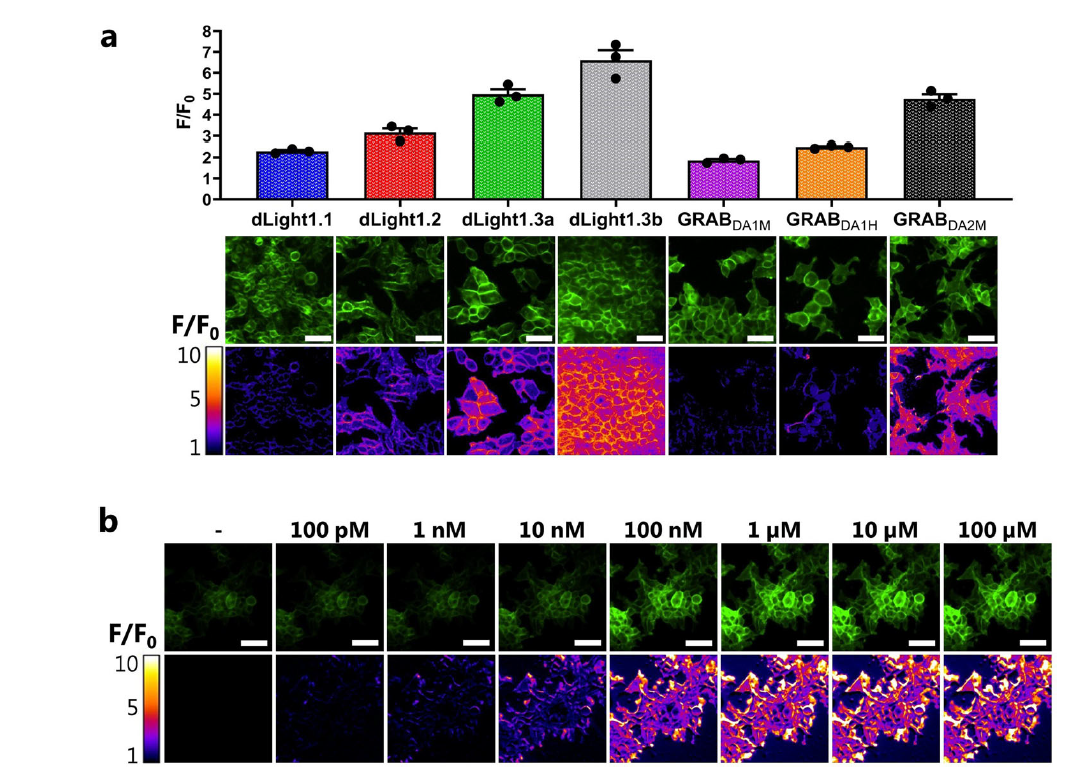 |
· Klein Herenbrink, C.#, Støier, J. F.#, Reith, W. D., Dagra, A., Gregorek, M. A. C., Cola, R. B., Patriarchi, T., Li, Y., Tian, L., Gether, U., & Herborg, F.* (2022). Multimodal detection of dopamine by sniffer cells expressing genetically encoded fluorescent sensors. Communications Biology, 5(1), 578.
[Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2021.09.16.460471 |
|
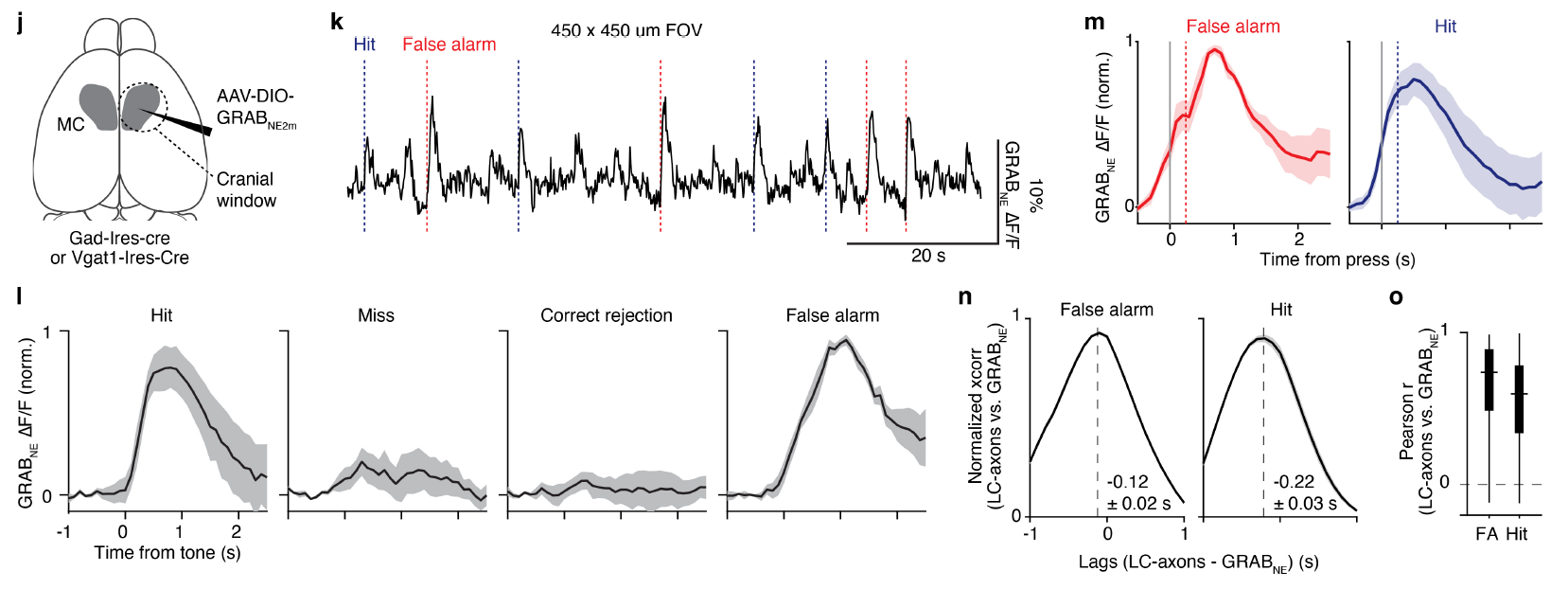 |
· Breton-Provencher, V.#*, Drummond, G. T.#, Feng, J., Li, Y., & Sur, M.* (2022).
Spatiotemporal dynamics of noradrenaline during learned behaviour. Nature [Full Text] [PDF] |
|
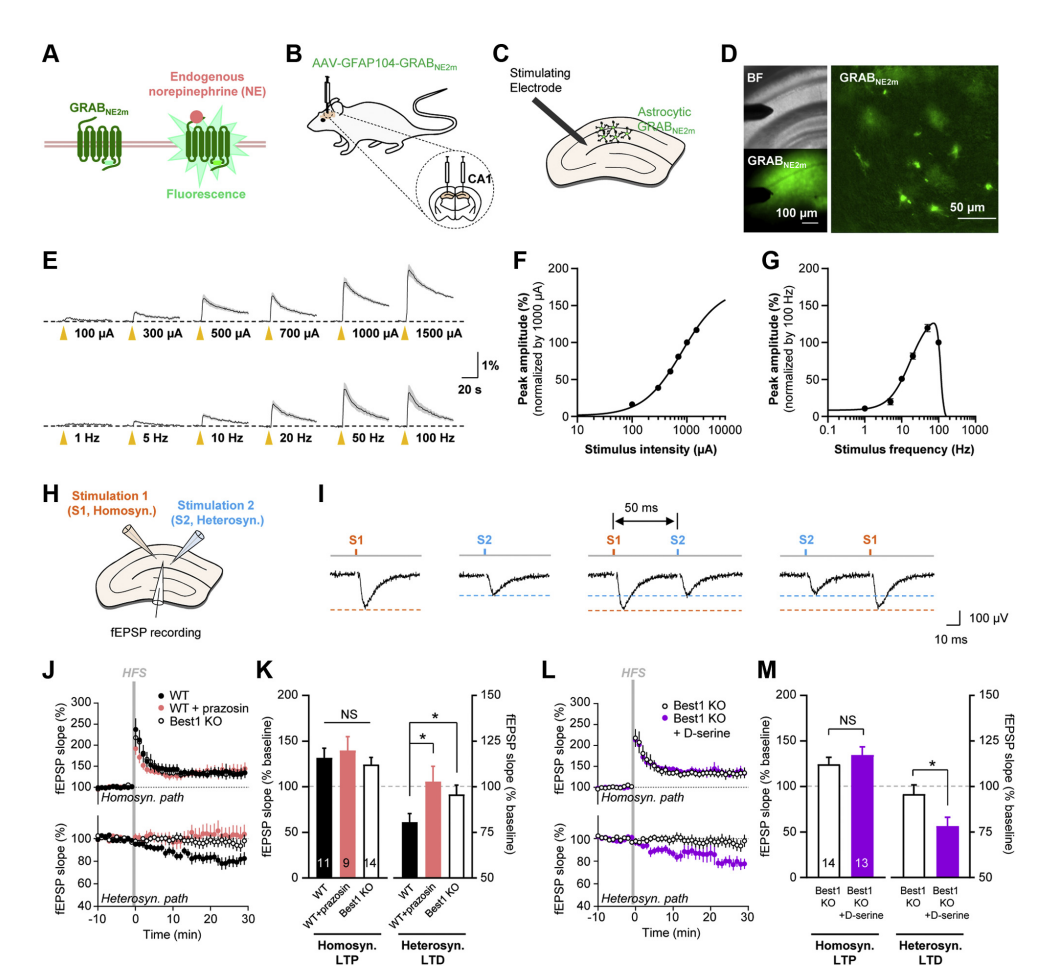 |
· Koh, W., Park, M., Chun, Y. E., Lee, J., Shim, H. S., Park, M. G., Kim, S., Sa, M., Joo, J., Kang, H., Oh, S.-J., Woo, J., Chun, H., Lee, S. E., Hong, J., Feng, J., Li, Y., Ryu, H., Cho, J., & Lee, C. J. (2021).
Astrocytes Render Memory Flexible by Releasing D-Serine and Regulating NMDA Receptor Tone in the Hippocampus.
Biological Psychiatry, 91(8), 740-752. [Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2021.03.25.436945 |
|
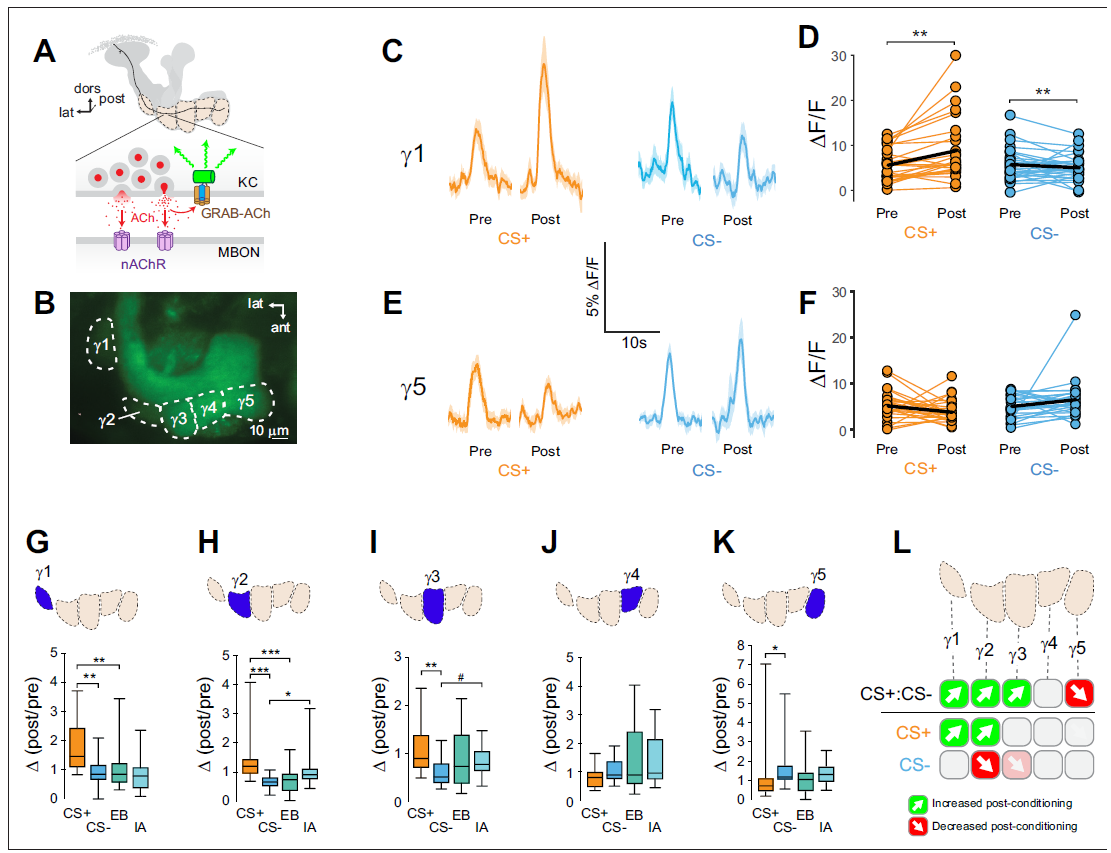 |
· Stahl, A., Noyes, N. C., Boto, T., Botero, V., Broyles, C. N., Jing, M., Zeng, J., King, L. B., Li, Y., Davis, R. L., & Tomchik, S. M.* (2022). Associative learning drives longitudinally graded presynaptic plasticity of neurotransmitter release along axonal compartments. eLife, 11.
[Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2021.06.08.447536 |
|
 |
· Liu, C.*, Cai, X., Ritzau-Jost, A., Kramer Paul, F., Li, Y.., Khaliq Zayd, M., Hallermann, S., & Kaeser Pascal, S.* (2022).
An action potential initiation mechanism in distal axons for the control of dopamine release. Science, 375(6587), 1378-1385. [Full Text] [PDF] * See Comments Highlight by: Wiseman, S. (2022).
Dopamine surprises.
Nature Neuroscience, 25(5), 531-531.
[Full Text] [PDF] |
|
.png) |
· Hasegawa, E., Miyasaka, A., Sakurai, K., Cherasse, Y., Li, Y.., & Sakurai, T.* (2022) Rapid eye movement sleep is initiated by basolateral amygdala dopamine signaling in mice.
Science, 375(6584), 994-1000. [Full Text] [PDF] |
|
 |
· Liput, D. J., Puhl, H. L., Dong, A., He, K., Li, Y., & Lovinger, D. M. (2022).
2-Arachidonoylglycerol mobilization following brief synaptic stimulation in the dorsal lateral striatum requires glutamatergic and cholinergic neurotransmission. Neuropharmacology, 205, 108916. [Full Text] [PDF] |
|
 |
· Deng, H.*, Xiao, X., Yang, T., Ritola, K., Hantman, A., Li, Y., Huang, Z. J., & Li, B.* (2021).
A genetically defined insula-brainstem circuit selectively controls motivational vigor.
Cell, 184(26), 6344-6360.e6318. [Full Text] [PDF] |
|
 |
· Hamilos, A. E., Spedicato, G., Hong, Y., Sun, F., Li, Y., & Assad, J. (2021). Slowly evolving dopaminergic activity modulates the moment-to-moment probability of reward-related self-timed movements.
eLife,, 10, e62583. [Full Text] [PDF] |
|
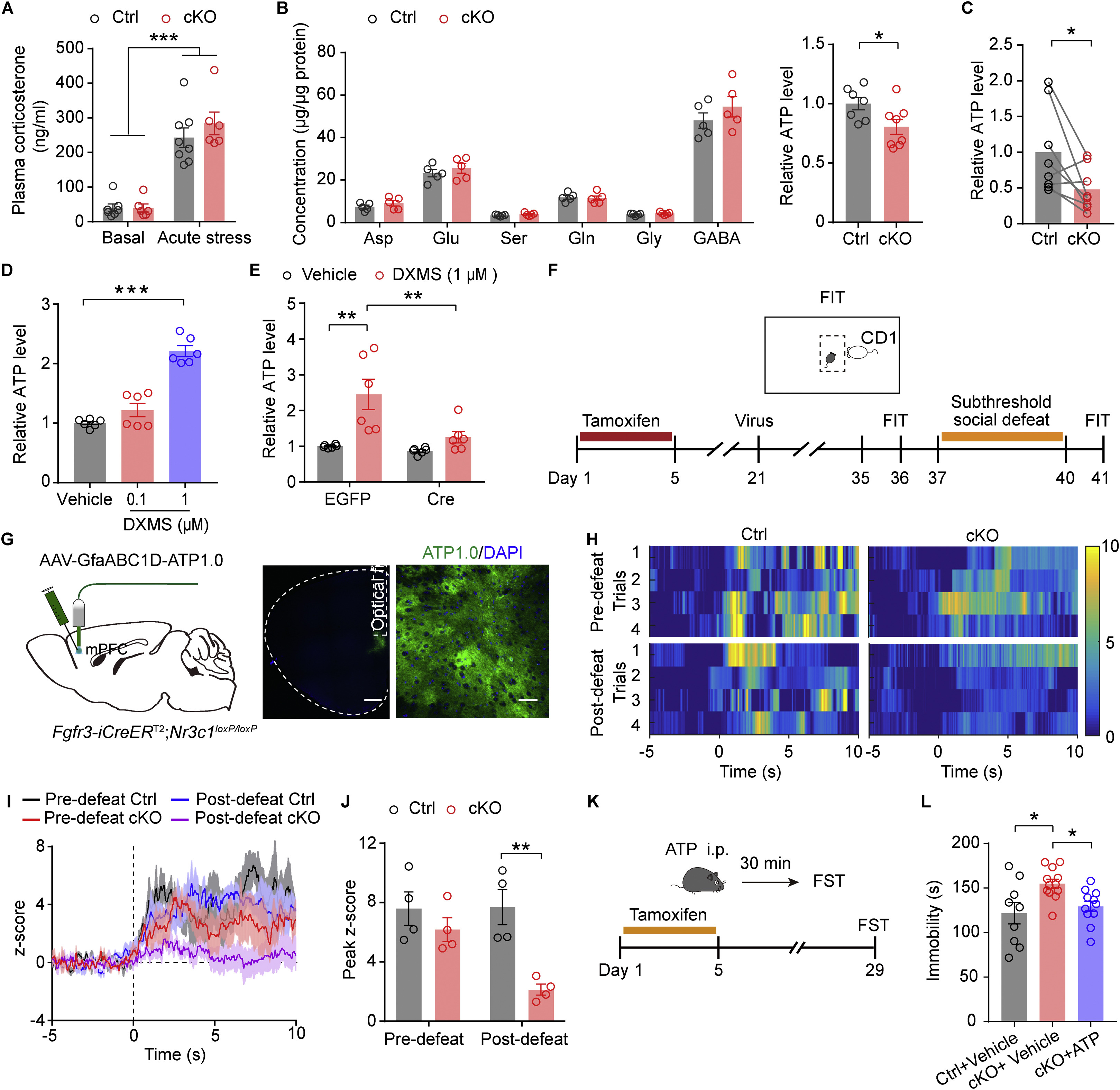 |
· Lu, C.-L.#, Ren, J.#, Mo, J.-W., Fan, J., Guo, F., Chen, L.-Y., Wen, Y.-L., Li, S.-J., Fang, Y.-Y., Wu, Z.-F., Li, Y., Gao, T.-M., & Cao, X.*(2021)
Glucocorticoid receptor-dependent astrocytes mediate stress vulnerability. Biological Psychiatry, 12(1), 6403. [Full Text] [PDF] |
|
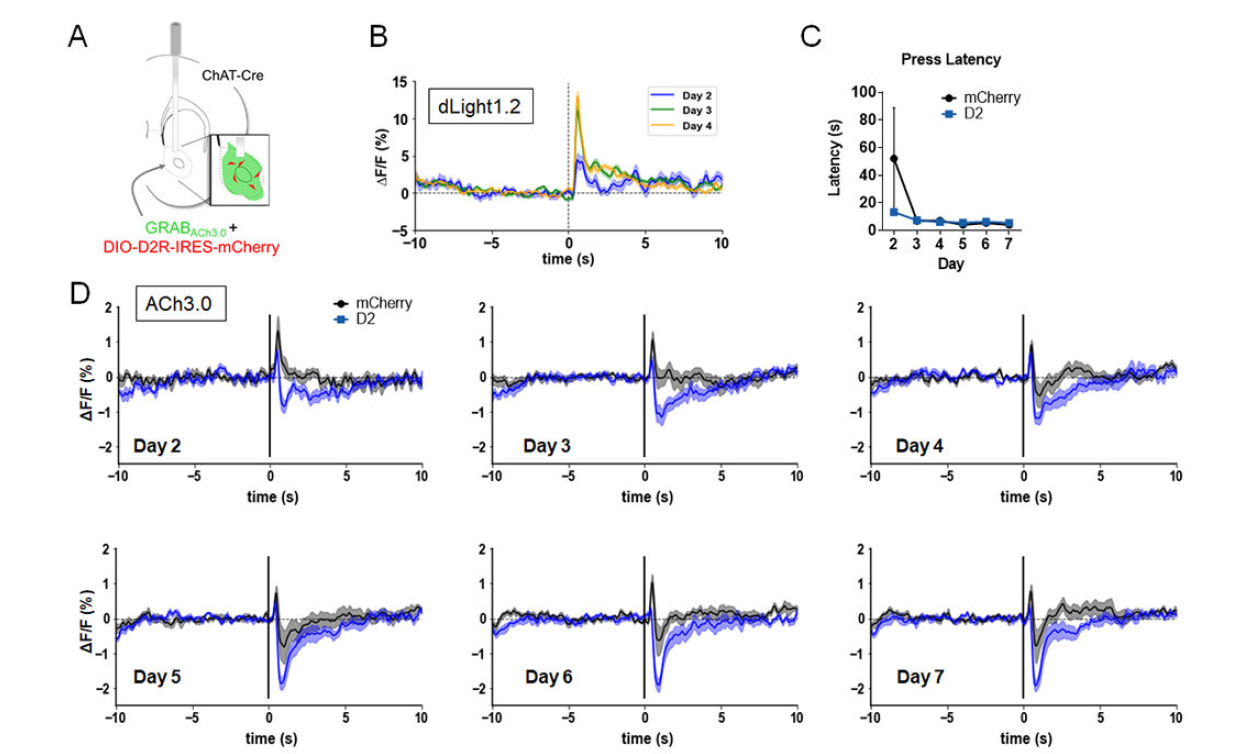 |
· Gallo, E. F.*, Greenwald, J., Yeisley, J., Teboul, E., Martyniuk, K. M., Villarin, Jing, M., Li, Y., Javitch, J. A., Balsam, P. D., & Kellendonk, C*. (2022).
Dopamine D2 receptors modulate the cholinergic pause and inhibitory learning. Mol Psychiatry, 27(3), 1502-1514. [Full Text] [PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2020.09.07.284612 |
|
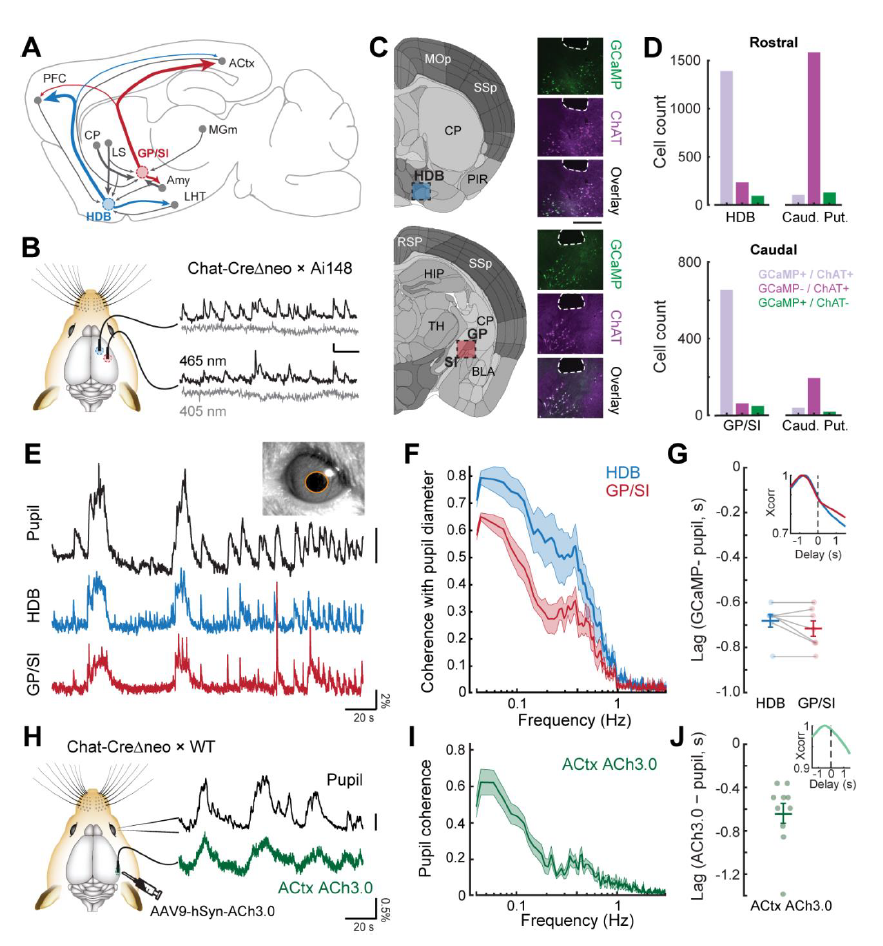 |
· Robert, B., Kimchi, E. Y., Watanabe, Y., Chakoma, T., Jing, M., Li, Y., & Polley, D. B. (2021). A functional topography within the cholinergic basal forebrain for encoding sensory cues and behavioral reinforcement outcomes. eLife, 10, e69514.
[Full Text] [PDF] |
|
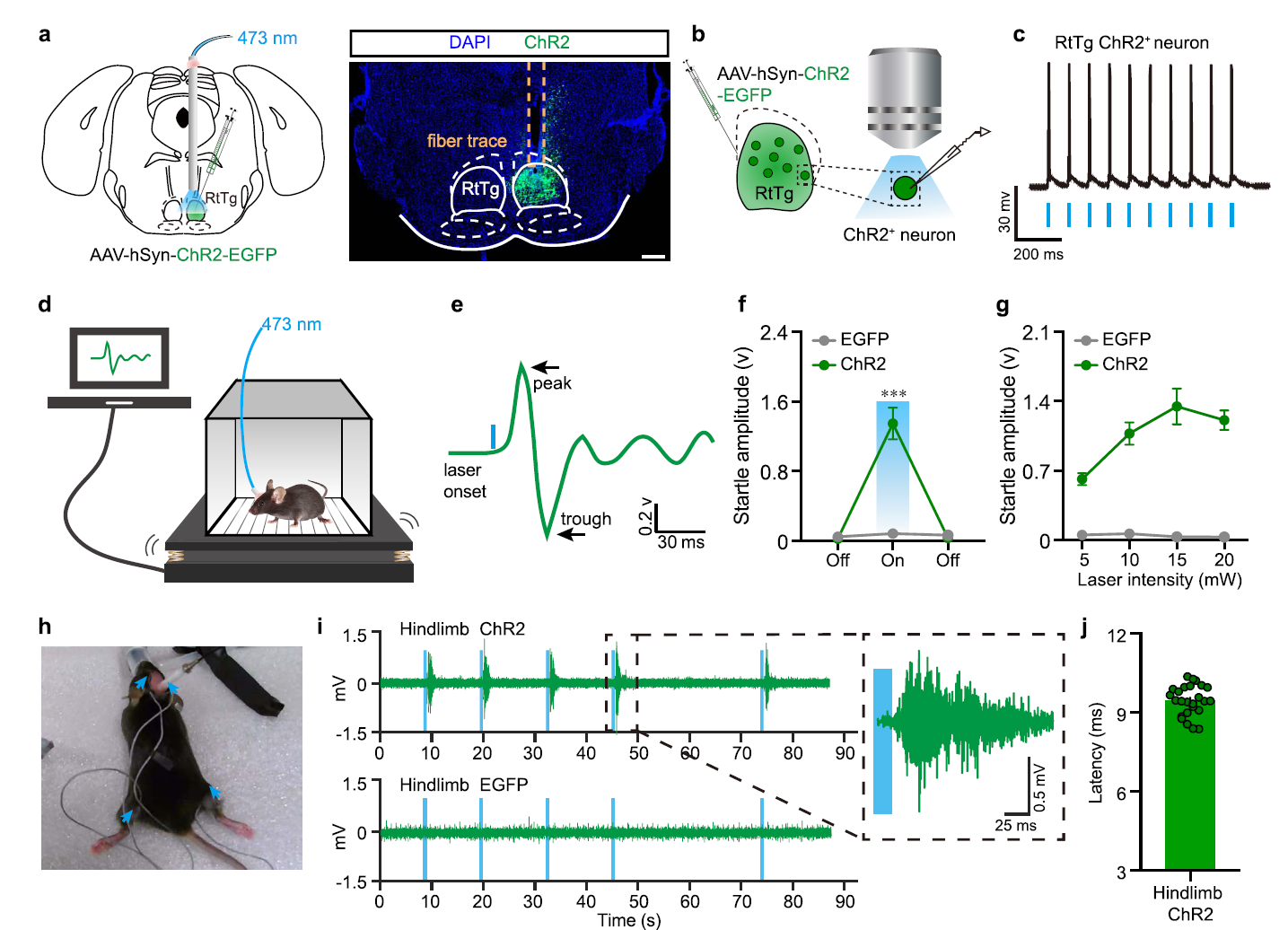 |
· Guo, W.#, Fan, S.#, Xiao, D., Dong, H., Xu, G., Wan, Z., Ma, Y., Wang, Z., Xue, T., Zhou, Y., Li, Y., & Xiong, W.* (2021).
A Brainstem reticulotegmental neural ensemble drives acoustic startle reflexes. Nature Communications, 12(1), 6403. [Full Text] [PDF] |
|
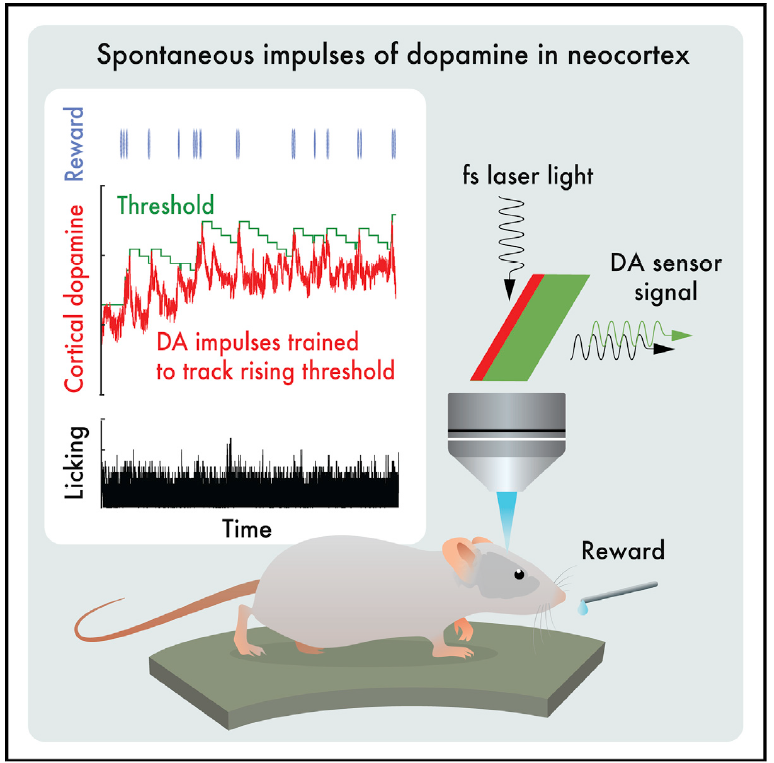 |
· Foo, C., Lozada, A., Aljadeff, J., Li, Y., Wang, J. W., Slesinger, P. A.*, & Kleinfeld, D.* (2021). Reinforcement learning links spontaneous cortical dopamine impulses to reward. Current Biology, 31(18), 4111-4119.e4114.
[Full Text] [PDF] |
|
 |
· Li, Y., Simmler Linda, D., Van Zessen, R., Flakowski, J., Wan, J.-X., Deng, F., Li, Y.-L., Nautiyal Katherine, M., Pascoli, V., & Lüscher, C.* (2021)
Synaptic mechanism underlying serotonin modulation of transition to cocaine addiction. Science, 73(6560), 1252-1256. [Full Text] [PDF] |
|
 |
· Al-Hasani, R.#*, Gowrishankar, R.#, Schmitz, G. P.#, Pedersen, C. E., Marcus, D. J., Shirley, S. E., Hobbs, T. E., Elerding, A. J., Renaud, S. J., Jing, M., Li, Y., Alvarez, V. A., Lemos, J. C., & Bruchas, M. R*. (2021). Ventral tegmental area GABAergic inhibition of cholinergic interneurons in the ventral nucleus accumbens shell promotes reward reinforcement. Nature Neuroscience, 24, 1414–1428
[Full Text] [PDF] |
|
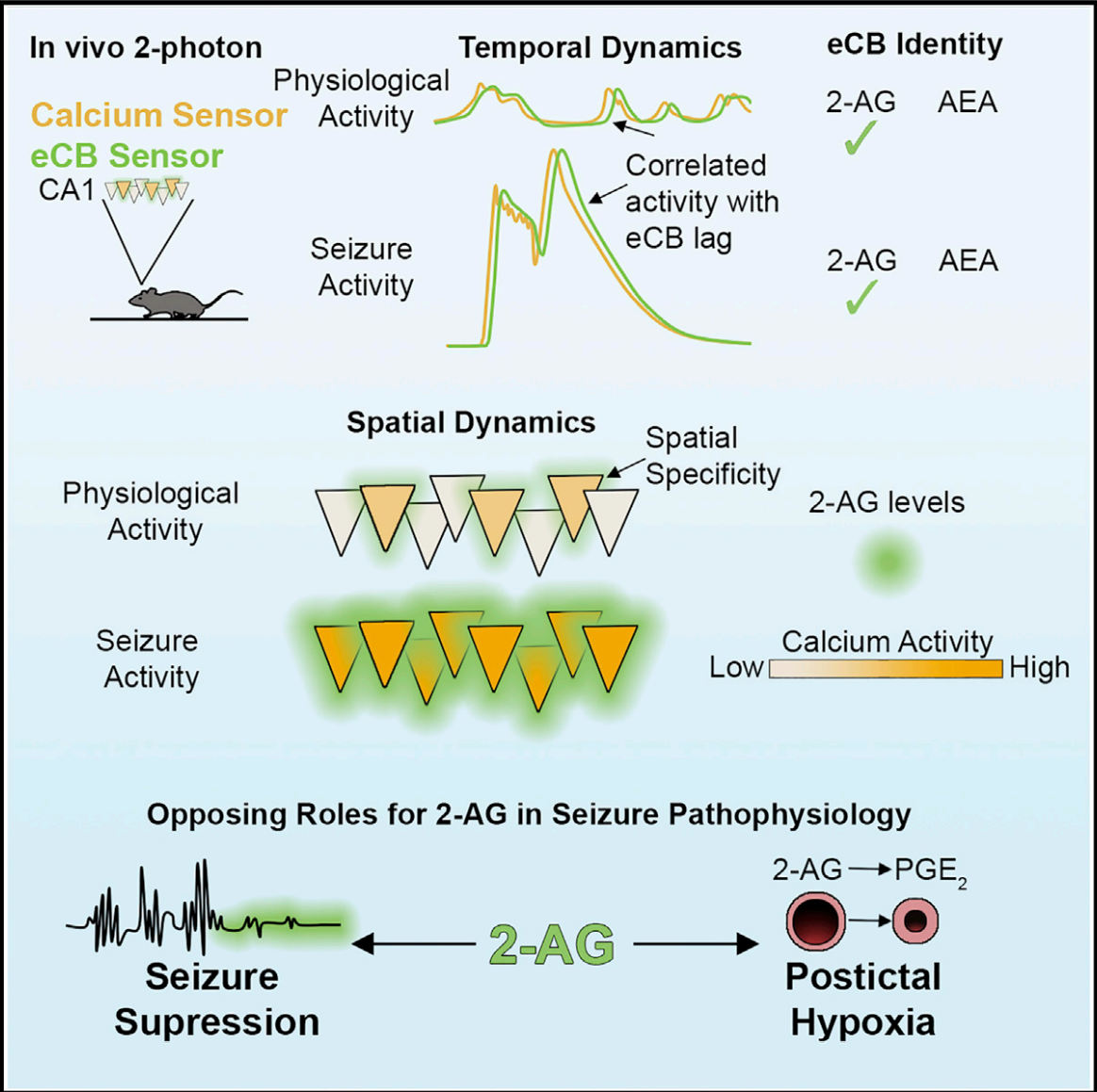 |
· Farrell, J. S.*, Colangeli, R., Dong, A., George, A. G., Addo-Osafo, K., Kingsley, P. J., Morena, M., Wolff, M. D., Dudok, B., He, K., Patrick, T. A., Sharkey, K. A., Patel, S., Marnett, L. J., Hill, M. N., Li, Y., Teskey, G. C., & Soltesz, I. (2021).
In vivo endocannabinoid dynamics at the timescale of physiological and pathological neural activity. Neuron,, 109(15), 2398-2403.e2394. [Full Text] [PDF] |
|
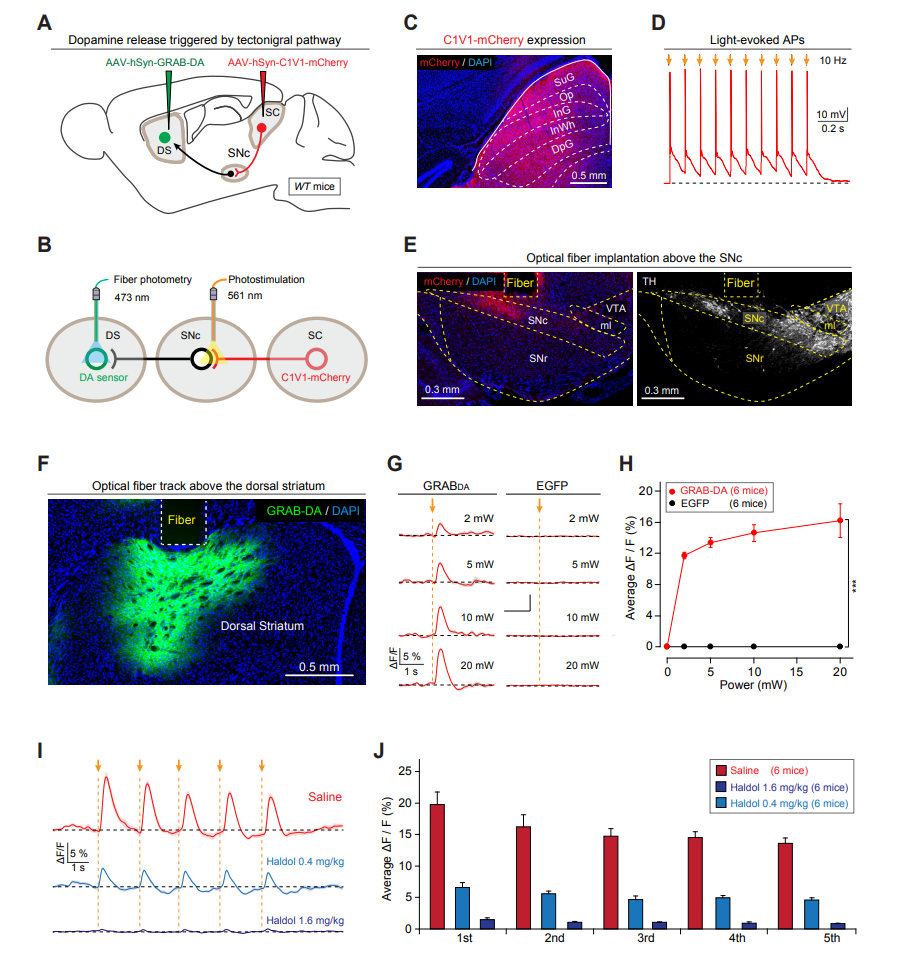 |
· Huang, M.#, Li, D.#*, Pei, Q.#, Xie, Z., Gu, H., Zhang, X., Chen, Z., Liu, A., Wang, Y., Sun, F., Li, Y., Zhang, J., He, M., Xie, Y., Zhang, F., Qi, X., Shang, C.*, & Cao, P.*(2021). The tectonigral pathway regulates appetitive locomotion in predatory hunting in mice Nature Communications, 12, 4409.
[Full Text] [PDF] |
|
 |
· Wang, Q.#, Kong, Y.#, Wu, D., Liu, J., Jie, W., You, Q., Huang, L., Hu, J., Chu, H., Gao, F., Hu, N., Luo, Z., Li, X., Li, S., Wu, Z., Li, Y., Yang, J.*, & Gao, T.* (2021).
Impaired calcium signaling in astrocytes modulates autism spectrum disorder-like behaviors in mice. Nature Communications, 12(1), 3321. [Full Text] [PDF] |
|
 |
· Pribiag, H., Shin, S., Wang, E. H., Sun, F., Datta, P., Okamoto, A., Guss, H., Jain, A., Wang, X. Y., De Freitas, B., Honma, P., Pate, S., Lilascharoen, V., Li, Y., & Lim, B. K.* (2021).
Ventral pallidum DRD3 potentiates a pallido-habenular circuit driving accumbal dopamine release and cocaine seeking. Neuron,
https://doi.org/10.1016/j.neuron.2021.05.002 [Full Text] [PDF] |
|
 |
· Zhang, Y.#, Cao, L.#, Varga, V., Jing, M., Karadas, M., Li, Y., & Buzsáki, G.* (2021). Cholinergic suppression of hippocampal sharp-wave ripples impairs working memory. Proceedings of the National Academy of Sciences, 118(15), e2016432118. https://doi.org/10.1073/pnas.2016432118. [Full Text] [PDF] |
|
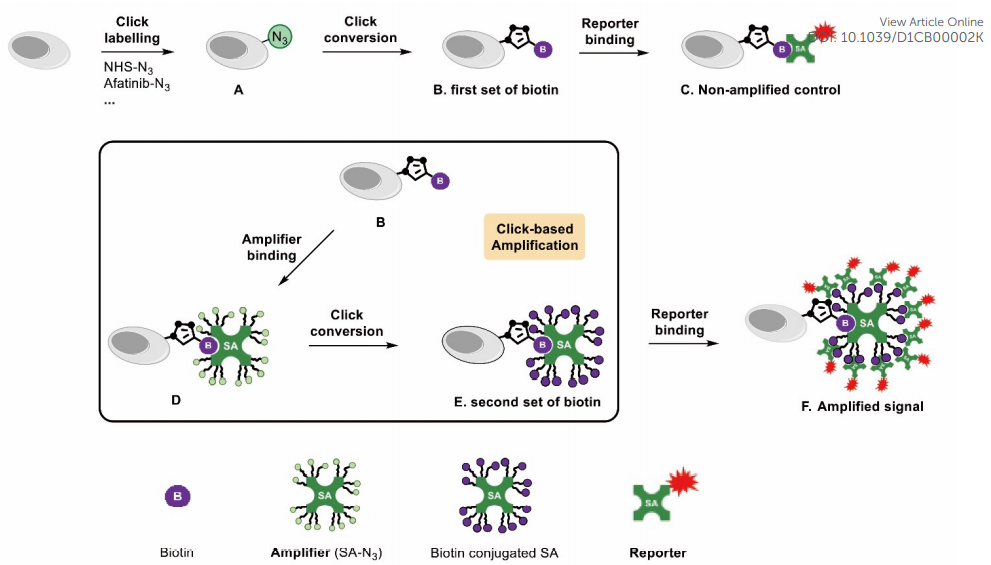 |
· Bai, J., Guo, F., Li, M., Li, Y.*, & Lei, X.* (2021). Click-based amplification: designed to facilitate various target labelling with ultralow background. RSC Chemical Biology,
https://doi.org/10.1039/D1CB00002K. [Full Text] [PDF] |
|
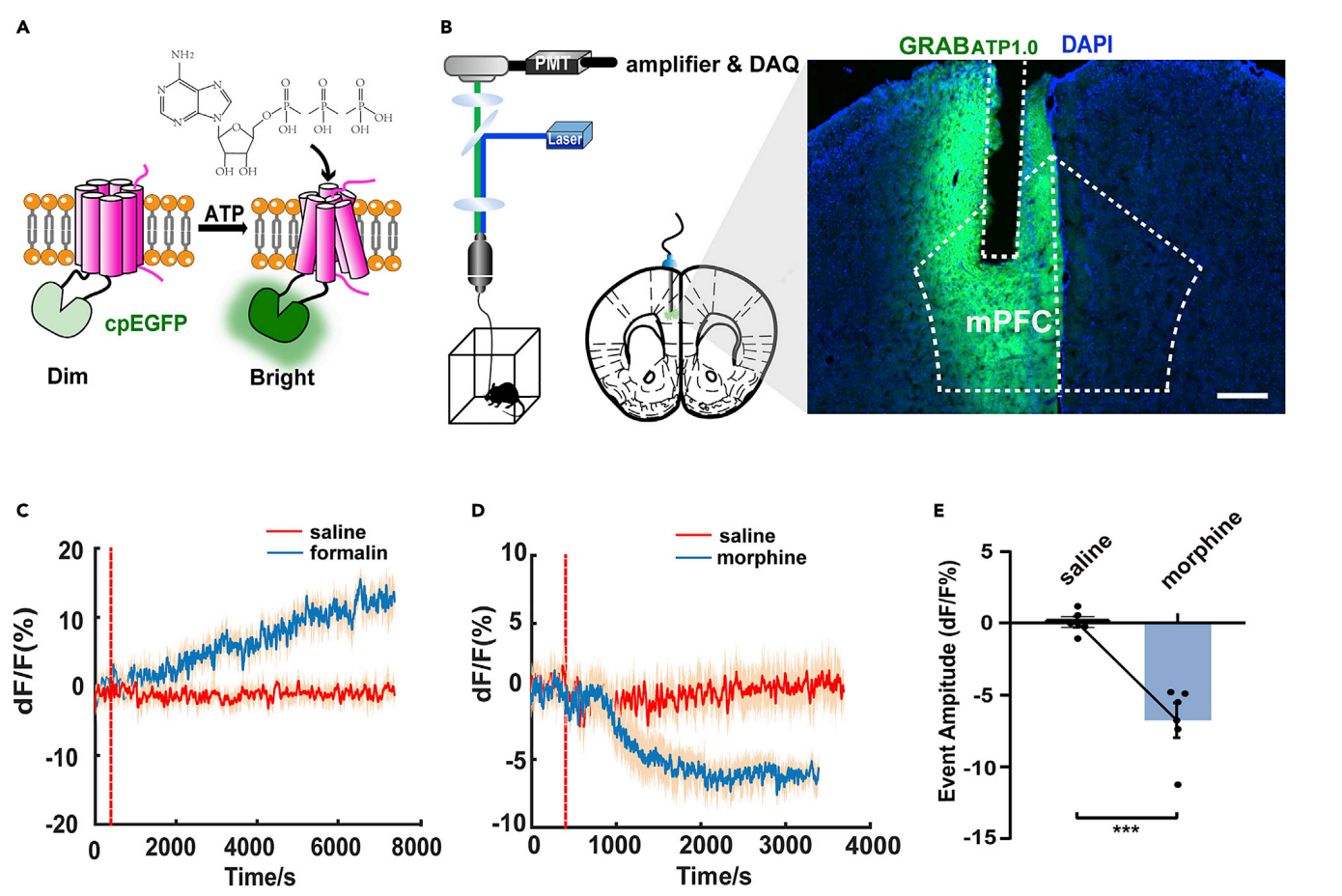 |
· Zeng, Y.#, Luo, H.#, Gao, Z., Zhu, X., Shen, Y., Li, Y., Hu, J.*, & Yang, J.* (2021). Reduction of prefrontal purinergic signaling is necessary for the analgesic effect of morphine. iScience,24(3), 102213. https://doi.org/https://doi.org/10.1016/j.isci.2021.102213. [Full Text] [PDF] |
|
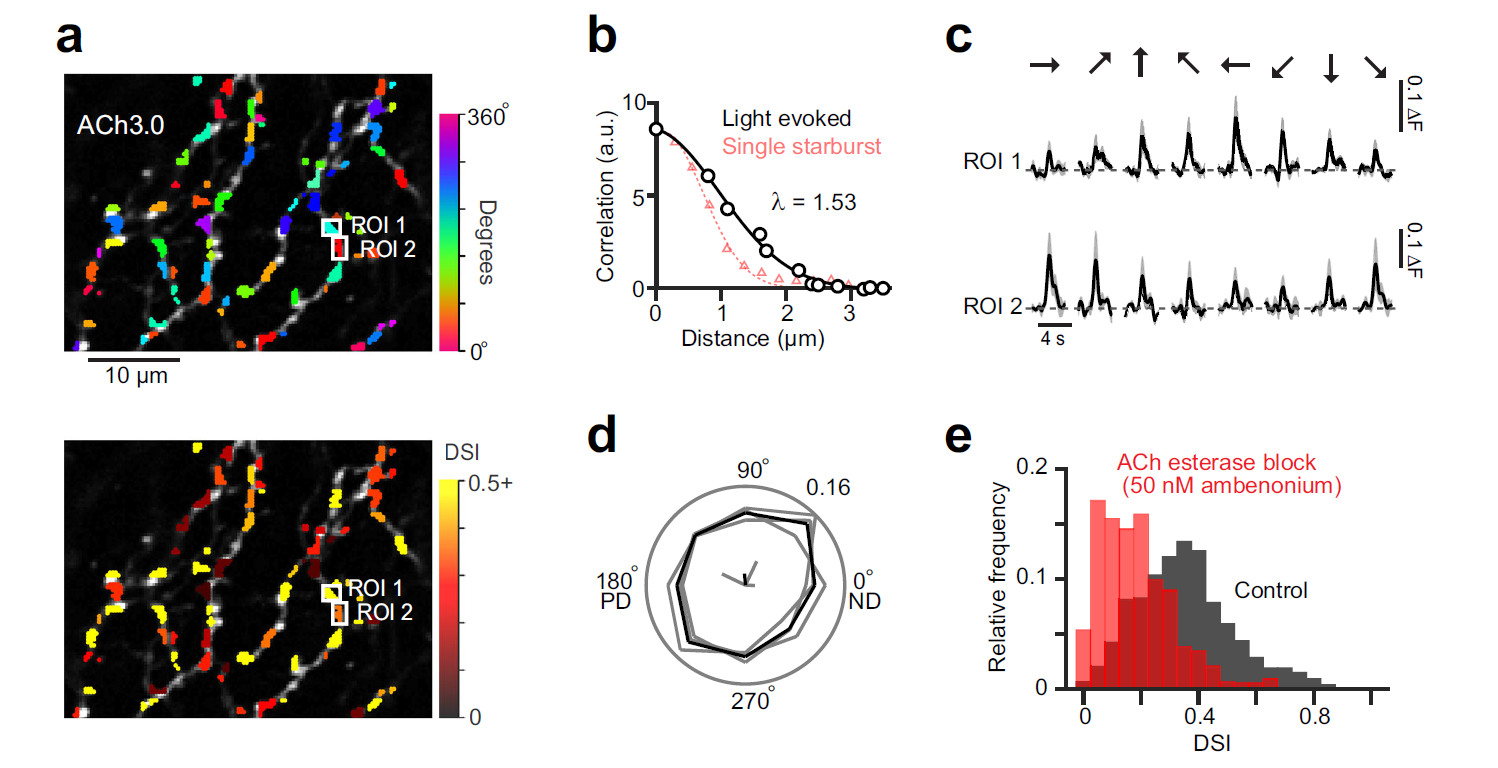 |
· Sethuramanujam, S.#, Matsumoto, A.#, deRosenroll, G., Murphy-Baum, B., McIntosh, J. M., Jing, M., Li, Y., Berson, D., Yonehara, K.*, & Awatramani, G. B.* (2021). Rapid multi-directed cholinergic transmission in the central nervous system. Nature Communications,
https://doi.org/10.1038/s41467-021-21680-9. [Full Text] [PDF] |
|
 |
· Wang, J.#, Li, J.#, Yang, Q.#, Xie, Y.-K., Wen, Y.-L., Xu, Z.-Z., Li, Y., Xu, T., Wu, Z.-Y., Duan, S., & Xu, H.* (2021). Basal forebrain mediates prosocial behavior via disinhibition of midbrain dopamine neurons. Proceedings of the National Academy of Sciences,118(7), e2019295118. https://doi.org/10.1073/pnas.2019295118. [Full Text] [PDF] |
|
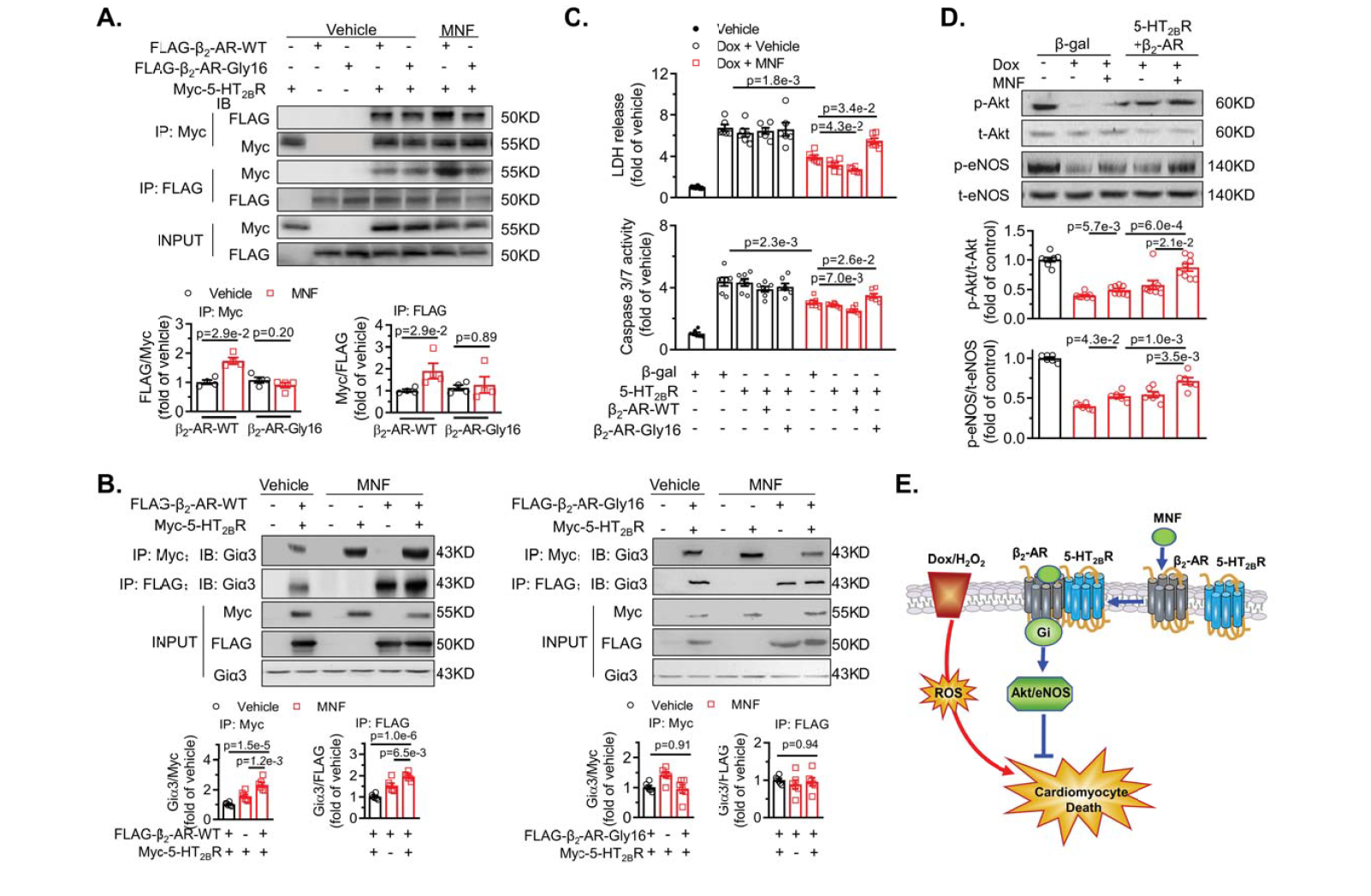 |
· Song, Y., Xu, C., Liu, J., Li, Y., Wang, H., Shan, D., Wainer Irving, W., Hu, X., Zhang, Y.*, Woo Anthony, Y.-H.*, & Xiao, R.-P. Heterodimerization with 5-HT2BR Is Indispensable for β2AR-mediated Cardioprotection. Circulation Research,
https://doi.org/10.1161/CIRCRESAHA.120.317011. [Full Text] [PDF] |
|
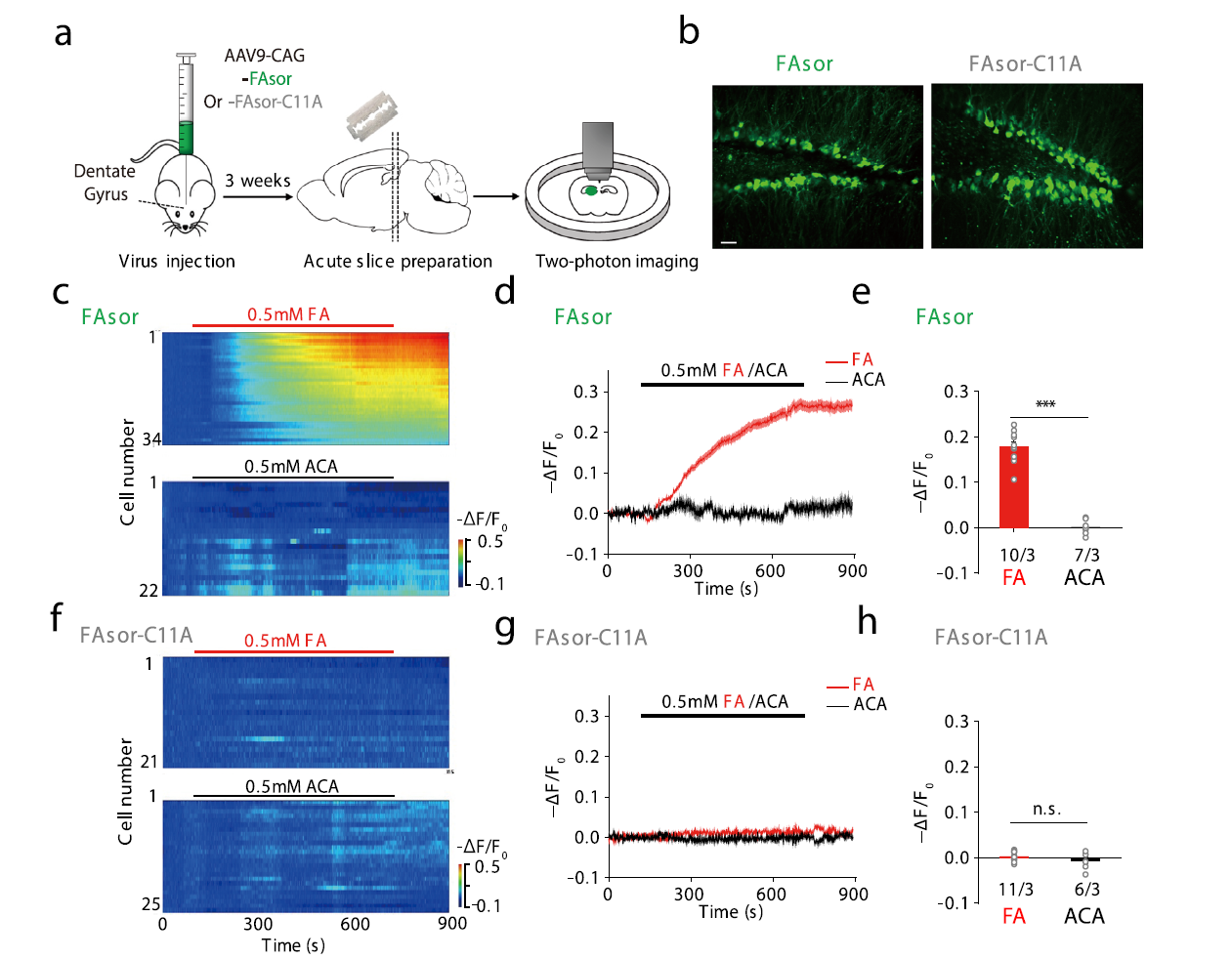 |
· Zhu, R.#, Zhang, G.#, Jing, M., Han, Y., Li, J., Zhao, J., Li, Y., & Chen, P. R.* (2021, 2021/01/25). Genetically encoded formaldehyde sensors inspired by a protein intra-helical crosslinking reaction. Nature Communications,,12(1), 581. https://doi.org/10.1038/s41467-020-20754-4. [Full Text] [PDF] |
|
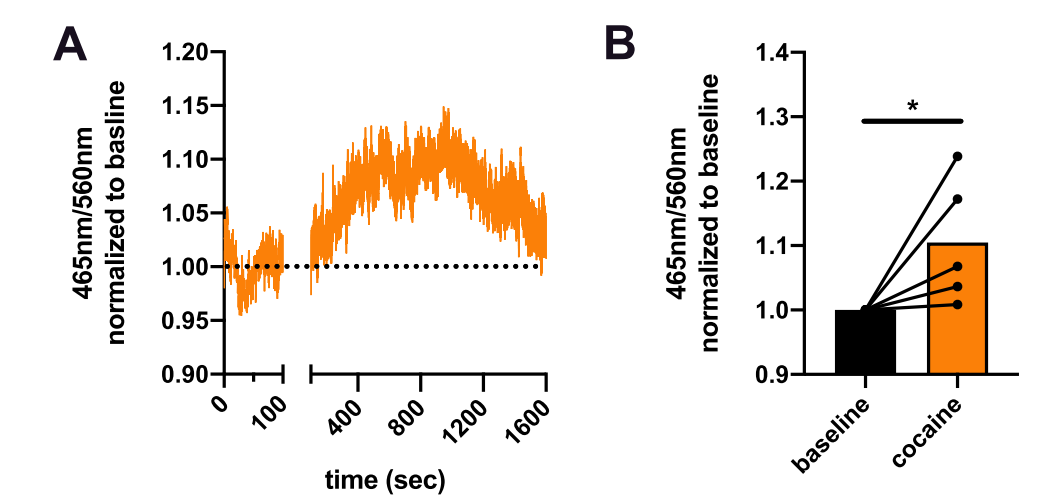 |
· Mayer, F. P., Iwamoto, H., Hahn, M. K., Grumbar, G. J., Stewart, A., Li, Y., & Blakely, R. D.* (2021). There's no place like home? Return to the home cage triggers dopamine release in the mouse nucleus accumbens. Neurochemistry International, 142, 104894. https://doi.org/https://doi.org/10.1016/j.neuint.2020.104894. [Full Text] [PDF] |
|
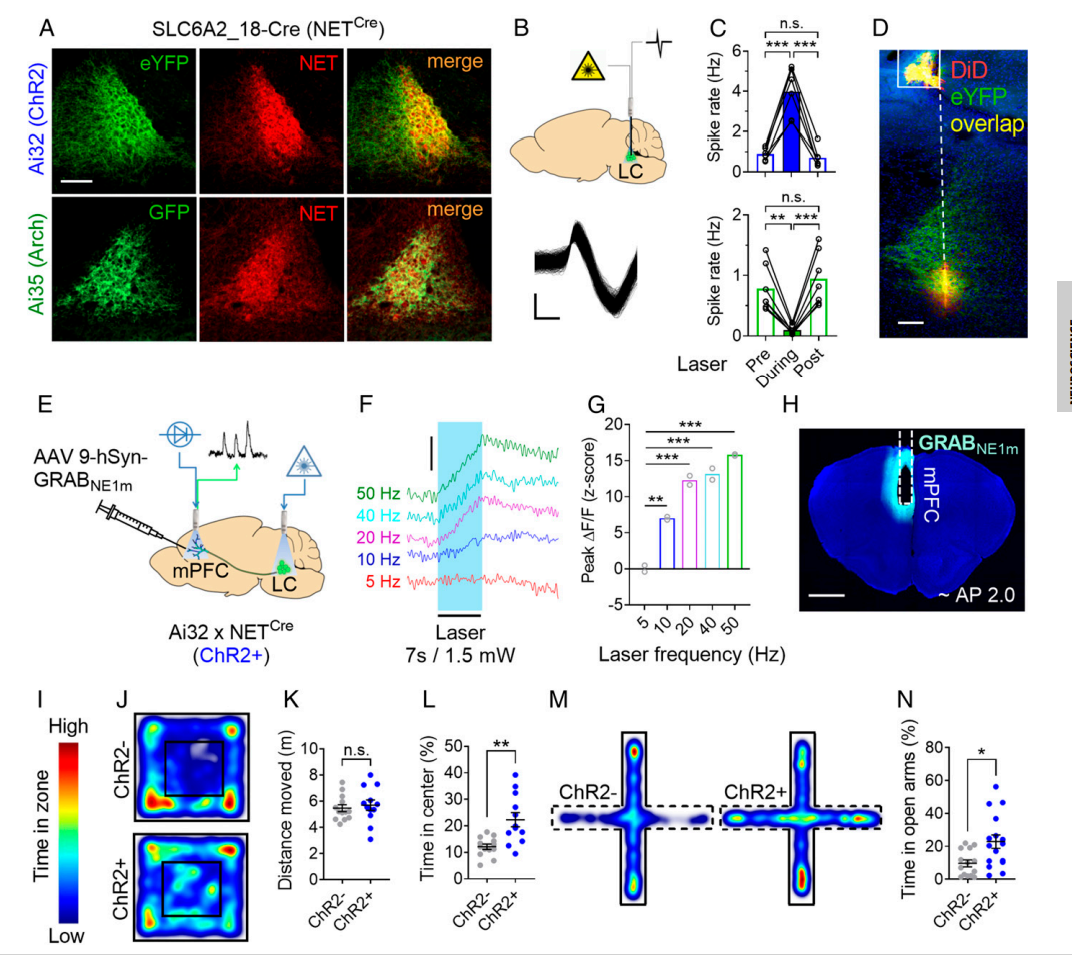 |
· Bari*, A., Xu, S., Pignatelli, M., Takeuchi, D., Feng, J., Li, Y., & Tonegawa, S.* (2020). Differential attentional control mechanisms by two distinct noradrenergic coeruleo-frontal cortical pathways. Proceedings of the National Academy of Sciences, https://doi.org/10.1073/pnas.2015635117. [Full Text] [PDF] |
|
 |
· Kim, H. R.*, Malik, A. N., Mikhael, J. G., Bech, P., Tsutsui-Kimura, I., Sun, F., Zhang, Y., Li, Y., Watabe-Uchida, M., Gershman, S. J., & Uchida, N.* (2020). A Unified Framework for Dopamine Signals across Timescales. Cell, https://doi.org/https://doi.org/10.1016/j.cell.2020.11.013. [Full Text] [PDF] |
|
 |
· Crouse,R. B., Kim, K., Batchelor, H. M., Kamaletdinova, R., Chan, J., Rajebhosale, P., Pittenger, S. T., Role, L. W., Talmage, D A., Jing, M. , Li, Y., Gao, X., Mineur , Y. S., & Picciotto, M. R. * (2020). Acetylcholine is released in the basolateral amygdala in response to predictors of reward and enhances learning of cue-reward contingency. eLife, 9:e57335. [Full Text] [PDF] |
|
 |
· Kwak, H., Koh, W., Kim, S., Song, K., Shin, J., Lee, J. M., Lee, E. H., Bae, J. Y., Ha, G. E., Oh, J. Park, Y. M., Kim, S., Feng, J., Lee, S. E., Choi, J. W., Kim, K. H., Kim, Y. S., Woo, J., Lee, D., Son, T., Kwon, S. W., Park, K. D., Yoon, B. Lee, J., Li, Y. , Lee, H., Bae, Y. C., Lee, C. J.* & Cheong, E.* (2020). Astrocytes Control Sensory Acuity via Tonic Inhibition in the Thalamus. Neuron, https://doi.org/10.1016/j.neuron.2020.08.013. [Full Text] [PDF] |
|
 |
· Peng, W.#, Wu, Z.#, Kun, S.#, Zhang, S., Li, Y. & Min, X.* (2020). Regulation of sleep homeostasis mediator adenosine by basal forebrain glutamatergic neurons. Science, 369, 1208. [Full Text] [PDF] |
|
 |
· Mazzone, C.M., Liang-Guallpa, J.,Li, C., Wolcott, N. S., Boone, M. H., Southern, M., Kobzar, N. P., Salgado, I. A., Reddy, D. M., Sun, F., Zhang, Y., Li, Y., Cui, G. * & Krashes, M. J.* (2020). High-fat food biases hypothalamic and mesolimbic expression of consummatory drives. Nature Neuroscience, https://doi.org/10.1038/s41593-020-0684-9. [Full Text] [PDF] |
|
 |
· DeGroot, S.R., Zhao-Shea, R., Chung L., Klenowski, P.M., Sun, F., Molas, S., Gardner, P.D., Li, Y. & Tapper, A.R.* (2020). Midbrain dopamine controls anxiety-like behavior by engaging unique interpeduncular nucleus microcircuitry. Biological Psychiatry, https://doi.org/10.1016/j.biopsych.2020.06.018. [Full Text] [PDF] |
|
 |
· Zhu, P. K. ,Zheng, W. S. , Zhang, P., Jing, M., Borden, P. M., Ali, F., Guo, K., Feng, J., Marvin, J. S., Wang, Y., Wan, J., Gan, L., Kwan, A. C., Lin, L., Looger, L. L., Li, Y. & Zhang, Y.* (2020). Nanoscopic visualization of restricted nonvolume cholinergic and monoaminergic transmission with genetically encoded sensors. Nano Lett., https://doi.org/10.1021/acs.nanolett.9b04877. [Full Text] [PDF] |
|
 |
· Lin, R.*, Liang, J., Wang, R., Yan, T., Zhou, Y., Liu, Y., Feng, Q., Sun, F., Li, Y., Li, A., Gong, H., & Luo, M.* (2020). The raphe dopamine system controls the expression of incentive memory. Neuron, 1420-19. [Full Text] [PDF] |
|
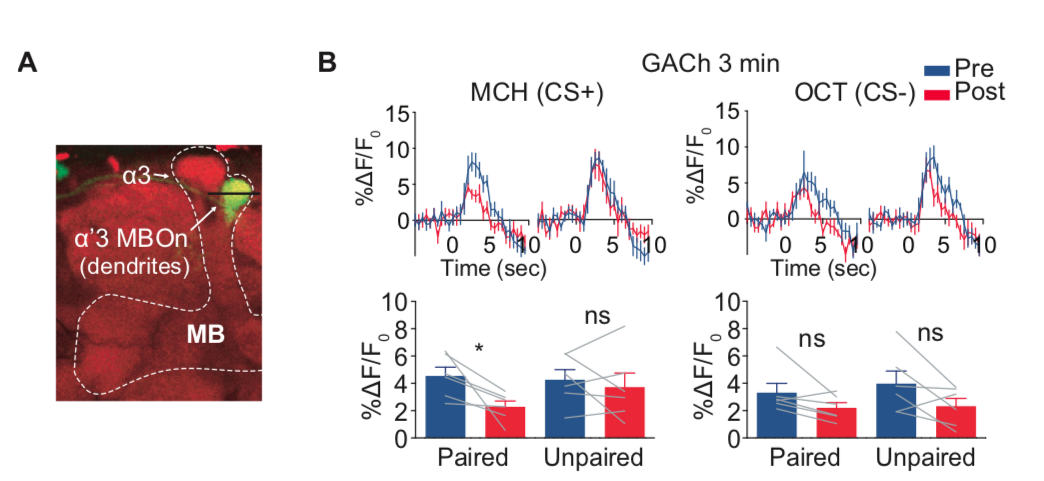 |
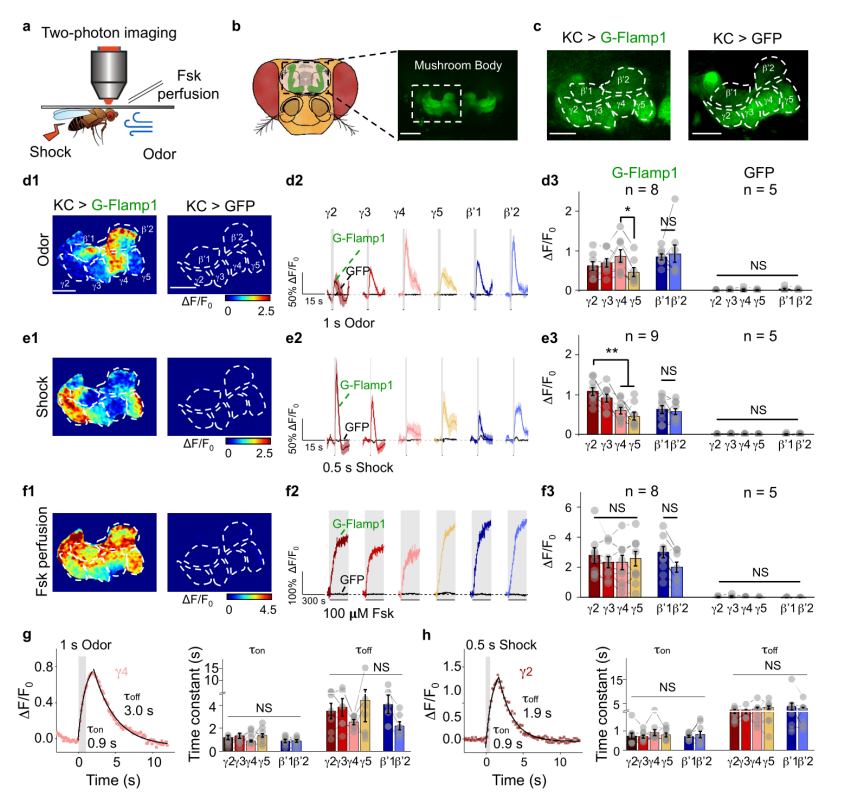
· Zhang, X., Noyes, N. C. , Zeng, J., Li, Y. & Davis, R. L.* (2019). Aversive training induces both pre- and postsynaptic suppression in Drosophila. The Journal of Neuroscience, 1420-19. [Full Text] [PDF] |
|
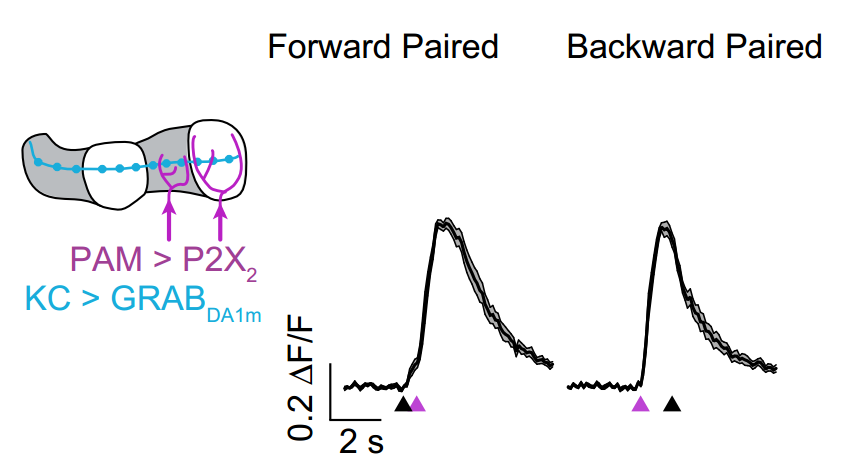 |
· Handler, A., Graham, T. G. M., Cohn, R., Morantte, I., Siliciano, A. F., Zeng J., Li, Y. & Ruta, V.* (2019). Distinct dopamine receptor pathways underlie the temporal sensitivity of associative learning. Cell, 178(1), 60-75. [Full Text] [PDF] |
|
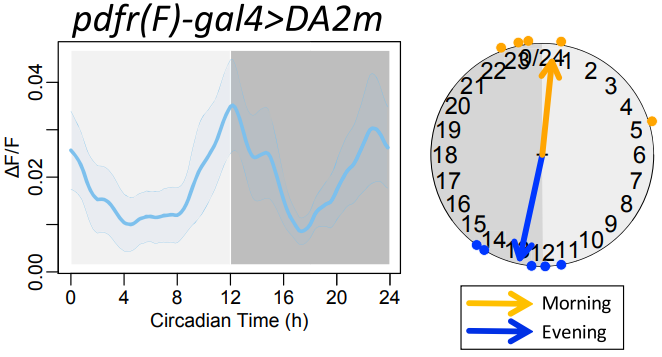 |
· Liang, X., Ho, M. C., Zhang, Y., Li, Y., Wu, M. N., Holy, T. E., & Taghert, P. H.* (2019). Morning and evening circadian pacemakers independently drive premotor centers via a specific dopamine relay. Neuron, 102(4), 843-857. [Full Text] [PDF] |
|
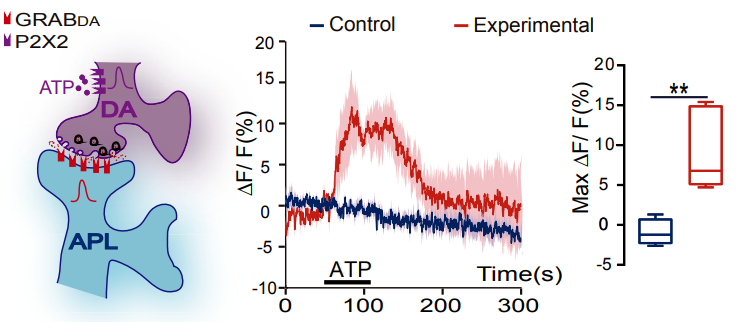 |
· Zhou, M.#, Chen, N.#, Tian, J., Zeng, J., Zhang, Y., Zhang, X., Guo, J., Sun, J., Li, Y., Guo, A.*, & Li, Y.* (2019). Suppression of GABAergic neurons through D2-like receptor secures efficient conditioning in Drosophila aversive olfactory learning. Proceedings of the National Academy of Sciences, 201812342. [Full Text] [PDF] |
|
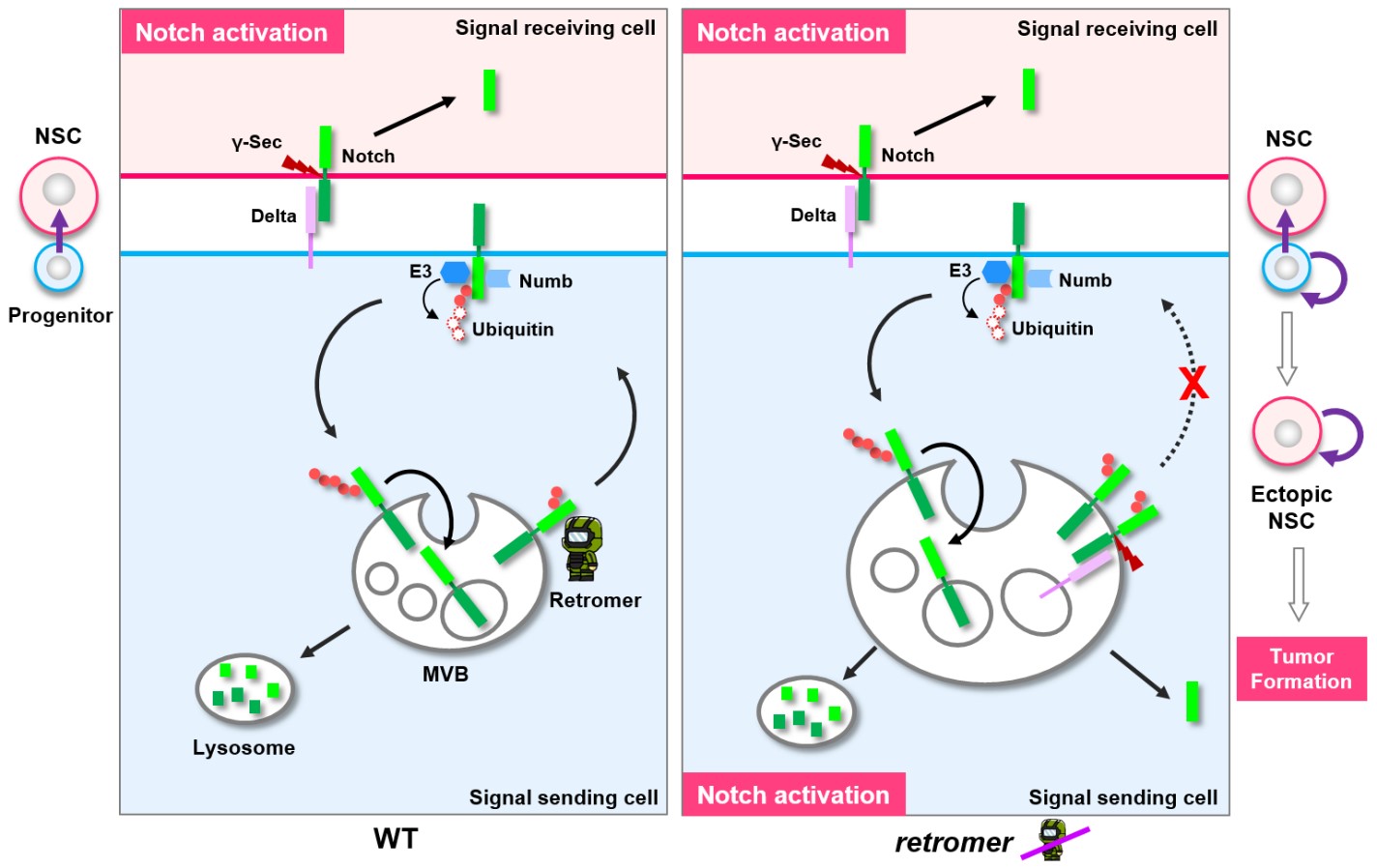 |
· Li, B.#, Wong, C.#, Gao, S. M., Zhang, R., Sun, R., Li, Y., & *Song, Y. (2018). The retromer complex safeguards against neural progenitor-derived tumorigenesis by regulating Notch receptor trafficking. eLife, 7, e38181. [Full Text] [PDF] |
|
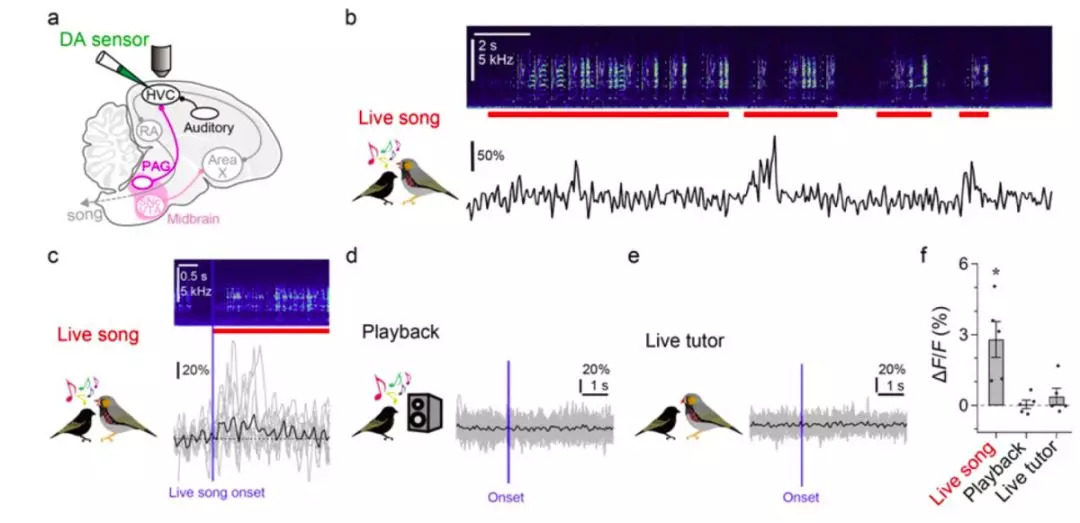 |
· Tanaka, M., Sun, F., Li, Y., & Mooney, R.* (2018). A mesocortical dopamine circuit enables the cultural transmission of vocal behaviour. Nature, 563(7729), 117-120. [Full Text] [PDF][Extended data][Supplementary information] |
|
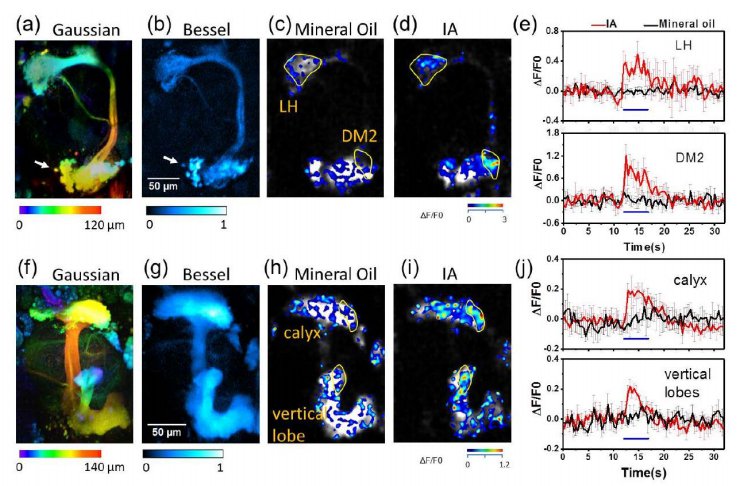 |
· Chen, B.#, Huang, X.#, Gou, D., Zeng, J., Chen , G., Pang, M., Hu, Y., Zhao, Z., Wu, H., Cheng, H., Zhang, Z., Xu, C., & Li, Y., Chen, L.*, Wang, A.* (2018). Rapid volumetric imaging with Bessel-Beam
three-photon microscopy. Biomedical optics express, 9(4), 1992-2000. [Full Text] [PDF] |
|
 |
· Shen, Y., Ge, W. P., Li, Y., Hirano, A., Lee, H. Y., Rohlmann, A., Missler, M., Tsien, R. W., Jan, L. Y., Fu, Y. H.* & Ptacek, L. J.* (2015). Protein mutated in paroxysmal dyskinesia interacts with the active zone protein RIM and suppresses synaptic vesicle exocytosis. Proceedings of the National Academy of Sciences, 112(10), 2935-2941. [Full Text] [PDF] |
|
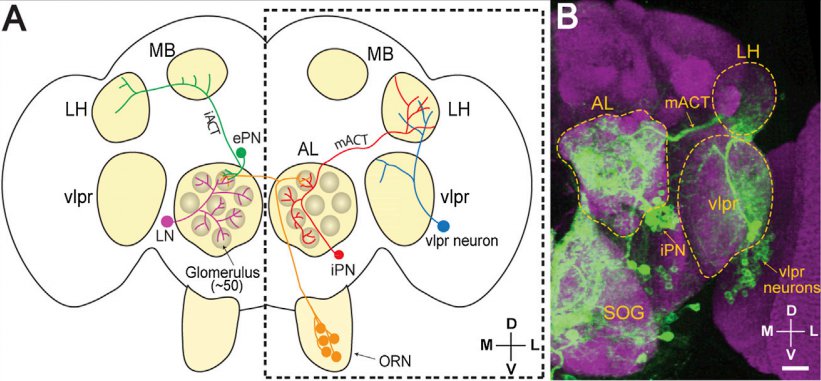 |
· Liang, L., Li, Y., Potter, C. J., Yizhar, O., Deisseroth, K., Tsien, R. W., & Luo, L.* (2013). GABAergic projection neurons route selective olfactory inputs to specific higher-order neurons. Neuron, 79(5), 917-931. [Full Text] [PDF] |
|
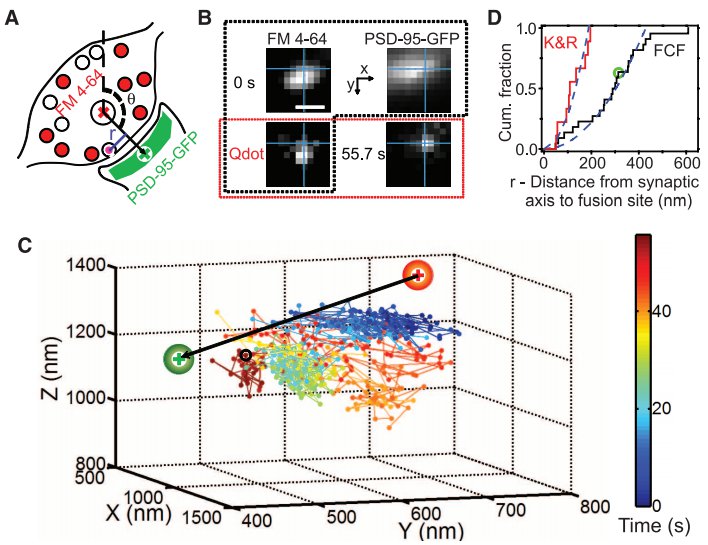 |
· Park, H., Li, Y., & Tsien, R. W.* (2012). Influence of synaptic vesicle position on release probability and exocytotic fusion mode. Science, 335(6074), 1362-1366. [Full Text] [PDF] |
|
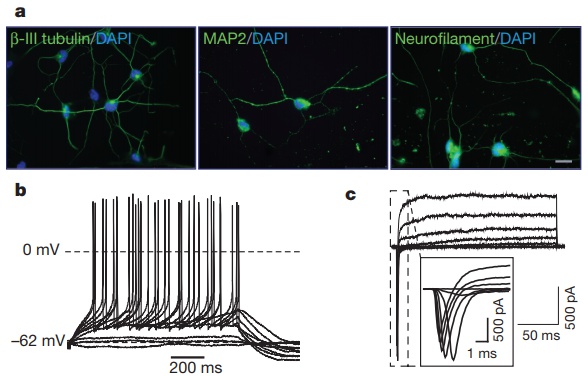 |
· Yoo, A. S.*, Sun, A. X., Li, L., Shcheglovitov, A., Portmann, T., Li, Y., Lee-Messer, C., Dolmetsch, R. E., Tsien R. W. & Crabtree, G. R.* (2011). MicroRNA-mediated conversion of human fibroblasts to neurons. Nature, 476(7359), 228-231. [Full Text] [PDF] |
|
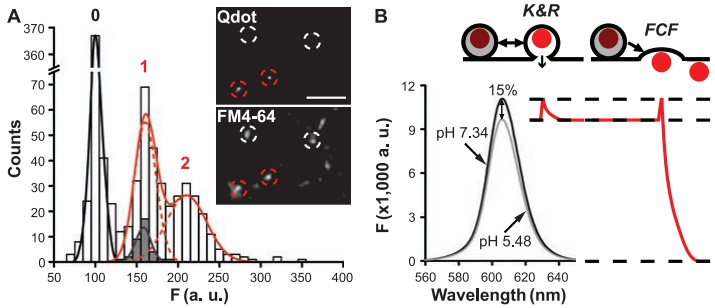 |
· Zhang, Q., Li, Y., & Tsien, R. W.* (2009). The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science, 323(5920), 1448-1453. [Full Text] [PDF] |
|
 |
· Kuner, T.*, Li, Y., Gee, K. R., Bonewald, L. F., & Augustine, G. J. (2008). Photolysis of a caged peptide reveals rapid action of N-ethylmaleimide sensitive factor before neurotransmitter release. Proceedings of the National Academy of Sciences, 105(1), 347-352. [Full Text] [PDF] |